Among the various programs created by the FDA, one stands out: the Voluntary Qualified Importer Program (VQIP). Notably, participation in this program offers numerous benefits for importers. So, what exactly is this program, and how can one register for it? In today’s article, UCC will provide you with a comprehensive overview and, therefore, answer all your questions regarding this special program.

1. Overview
The Voluntary Qualified Importer Program (VQIP) is an initiative by the FDA designed to expedite the review and importation process for human and animal food into the United States. By participating in VQIP, importers can avoid unexpected issues during the import process and ensure quicker product clearance. This contributes to securing the supply chain and benefits consumers by maintaining food safety and security.

To join, importers must meet the eligibility criteria and pay the associated fees. The fees cover the FDA’s program management costs. Furthermore, importers interested in registration may create an account on FDA’s Industry Systems. Once registered, they should select VQIP within the FSMA program options. Subsequently, they must submit an Intent to Participate through the VQIP Registration Page. Moreover, for those seeking registration for the upcoming benefit period, we strongly recommend referring to the VQIP User Guide.
2. Benefits of Participating in the Voluntary Qualified Importer Program
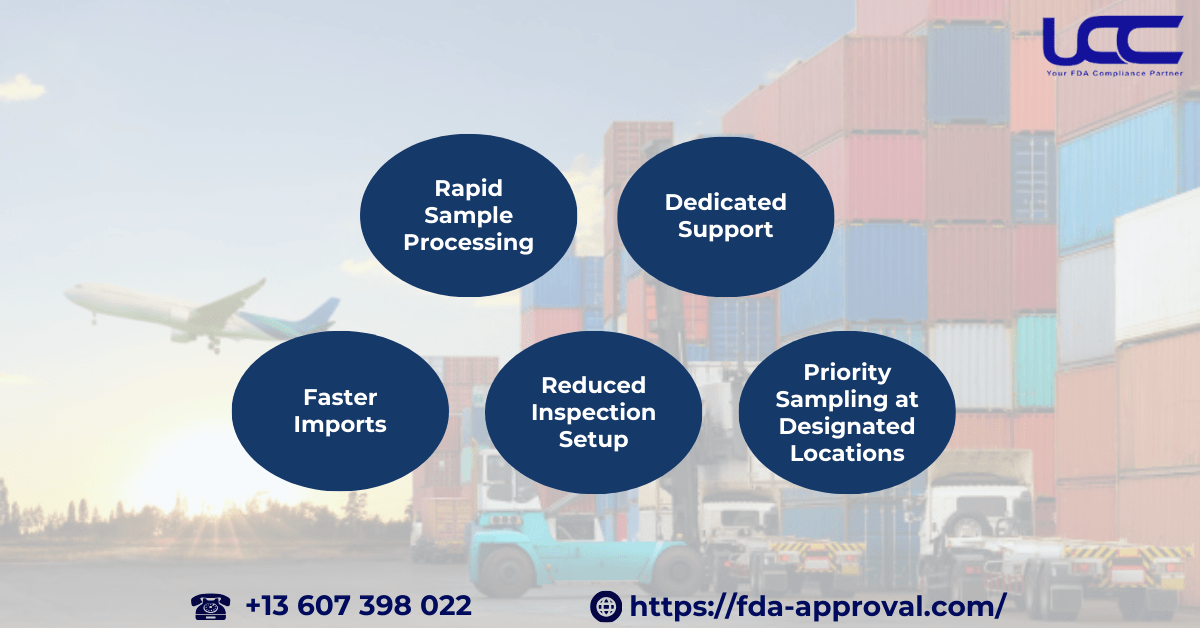
Participating in VQIP offers several advantages for importers:
- Faster Imports: Products covered by the Voluntary Qualified Importer Program application will be expedited through customs clearance into the U.S., speeding up the import process.
- Reduced Inspection Setup: The FDA limits inspections and sampling of VQIP shipments, performing them only when necessary, such as during outbreak investigations or random sampling.
- Priority Sampling at Designated Locations: When inspections are required, FDA will prioritize sampling at locations designated by the importer, minimizing disruptions.
- Rapid Sample Processing: FDA will prioritize processing samples from VQIP shipments in their labs, which shortens the time needed to obtain results.
- Dedicated Support: Importers can contact the VQIP Help Desk, where FDA staff are ready to resolve inquiries and issues during the import process.
3. Eligibility Criteria for the Voluntary Qualified Importer Program
To qualify for VQIP, importers must meet several requirements:
- Import Experience: Importers must have at least three years of experience in importing food products into the U.S., covering all types of food.
- DUNS Number: A Data Universal Numbering System (DUNS) number is mandatory for registration.
- Broker/Electronic Filer: Importers must use a well-rated FDA broker/electronic filer.
- No Import Alerts/Recalls: Importers must ensure that the products they are importing are not subject to import alerts or recalls when applying.
- No Ongoing FDA Administrative Actions: The FDA requires that neither the importer nor related parties be involved in ongoing administrative actions or legal proceedings. Additionally, they must not have a history of non-compliance with food safety regulations.
- FSVP/HACCP Compliance: The importer or their suppliers must comply with FSVP (Foreign Supplier Verification Program) or HACCP (Hazard Analysis and Critical Control Points) regulations.
- Supplier Certifications: Importers must provide certifications for the current facilities of foreign suppliers.
- VQIP Quality Assurance Program (QAP): Importers must develop and submit a Quality Assurance Program (QAP) with their application.
- No Customs Penalties: Importers must not have any penalties or seizures by U.S. Customs related to their imported products.
- Annual Use Fees: Importers must pay the annual fees before October 1 each year.
4. Application Requirements
4.1. Timeline
You can submit the Intent to Participate in Voluntary Qualified Importer Program between January 1 and May 31, by 11:59 PM EST. Moreover, this submission must be made through the FDA Industry Systems website.

4.2. Required Information
Importers must provide the following details during the application:
- Importing Company Identification Information: This includes the company’s name, address, tax identification number (if applicable), and contact information. Consequently, this ensures that the FDA can accurately identify and manage the food importation process.
- FSVP Contact Information: This is for the Foreign Supplier Verification Program, which requires importers to verify foreign suppliers to ensure their food meets FDA’s safety standards.
- Voluntary Qualified Importer Program Quality Assurance Program (QAP) Documentation: This program ensures that food imports meet quality and safety standards. Specifically, importers must provide evidence that their quality control systems align with FDA guidelines.
- Broker/Filer Contact Information: Importers must provide contact information for their customs broker or filer, who is responsible for handling customs procedures and food importation.
- Foreign Supplier Contact Information and Food List: Details about foreign suppliers (including facilities or food providers) and a list of food products intended for import. This allows FDA to verify that suppliers comply with safety standards.
For more information: Preventive Controls for Human Food: Compliance Essentials
By reading this article, you should now understand the requirements and criteria to register for the VQIP program. UCC hopes your business will thrive and continue to grow stronger each day! We look forward to sharing more valuable insights in future articles to help your business grow and succeed.



