The Public Health Security and Bioterrorism Preparedness and Response Act requires the owner, operator, or agent in charge of a domestic or foreign facility that performs manufacturing, processing, or packaging , storing food for human or animal consumption in the United States must register the facility with the FDA.
A domestic facility is any facility located in any State or Territory of the United States, the District of Columbia, or the Commonwealth of Puerto Rico. A foreign facility is an establishment that is not located in the United States. Registration covers only establishments – domestic and foreign engaged in the production, processing, packaging or storage of food for consumption in the United States.
There are a number of other establishments that do not have to register because they do not produce, process, package, or preserve food. Facilities that manufacture, process, package, or simply store food contact substances or pesticides are not required to register.
The food facility registrant submits additional registration information to FDA, including the contact person’s email address for domestic facilities, the U.S. agent’s email address for foreign facilities. Food establishments need to renew their registration with the FDA every two years; Failure to do so will result in the registration being cancelled. Specifically, during the period starting from October 1 and ending on December 31 every year, food establishments that have submitted documents to the FDA must submit to the FDA an extension of registration.
FDA may suspend the registration of a food facility under certain circumstances if FDA determines that the food produced, processed, packaged, received, or held by the registered facility is likely to cause harm. cause serious health consequences or death in humans or animals.
Consequences of not registering with the FDA: US law clearly stipulates that goods imported into the US without registering with the FDA will violate Federal Law and the US government can prosecute businesses according to the law. this career. In case this is a foreign goods imported into the US, if there is a violation, all goods will be detained at the port under the management of FDA & CBP (Customs and Border Protection) and handled according to regulations. provisions of law and federal regulations.
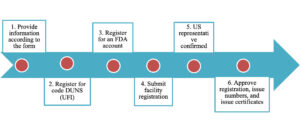
The process for registering a food facility under the FDA is as follows:

Responsibilities of a US FDA Agent
The US FDA Agent must either reside in the US or maintain a place of business in the US. The US FDA Agent cannot use a post office box as an address. The US FDA Agent cannot use just an answering service. They must be available to answer the phone or have an employee available to answer the phone during normal business hours.
The responsibilities of the US FDA Agent are limited and include:
-
Assisting FDA in communications with the foreign establishment.
-
Responding to questions concerning the foreign establishment’s devices that are imported or offered for import into the United States.
-
Assisting FDA in scheduling inspections of the foreign establishment.
-
If FDA is unable to contact the foreign establishment directly or expeditiously, FDA may provide information or documents to the US FDA Agent, and such an action shall be considered to be equivalent to providing the same information or documents to the foreign establishment.



