Medical devices are indispensable tools in the diagnosis and treatment of patients, significantly enhancing healthcare outcomes. They not only reduce the time required for examinations and treatments but also enhance efficiency and improve patient health outcomes. If you are involved in the business of medical devices and aim to enter the U.S. market, it is essential to register annually with the U.S. Food and Drug Administration (FDA). This article aims to clarify key information regarding how to register a medical device with the FDA, with UCC providing essential guidance.

1. Overview
The initial regulations for medical devices in the United States emerged in 1976 and have, over time, evolved significantly. Currently, these regulations are managed by the Center for Devices and Radiological Health (CDRH) within the FDA. In addition, with stringent regulations and rigorous requirements, the FDA plays a critical role in protecting and promoting public health.
Furthermore, any facility aiming to manufacture, import, reprocess, or develop specifications for medical devices or in vitro diagnostics (IVD) intended for commercial sale in the U.S. is required to register each year with the FDA. This process is known as “Establishment Registration” and is, importantly, distinct from the 510(k) submission or Premarket Approval (PMA) process.
2. Who Must Register Medical Device with the FDA?
As a general principle, if you manufacture any part of a medical device sold in the U.S. or perform processing activities (such as sterilization), you must register with the FDA.
3. FDA Classification of Medical Devices
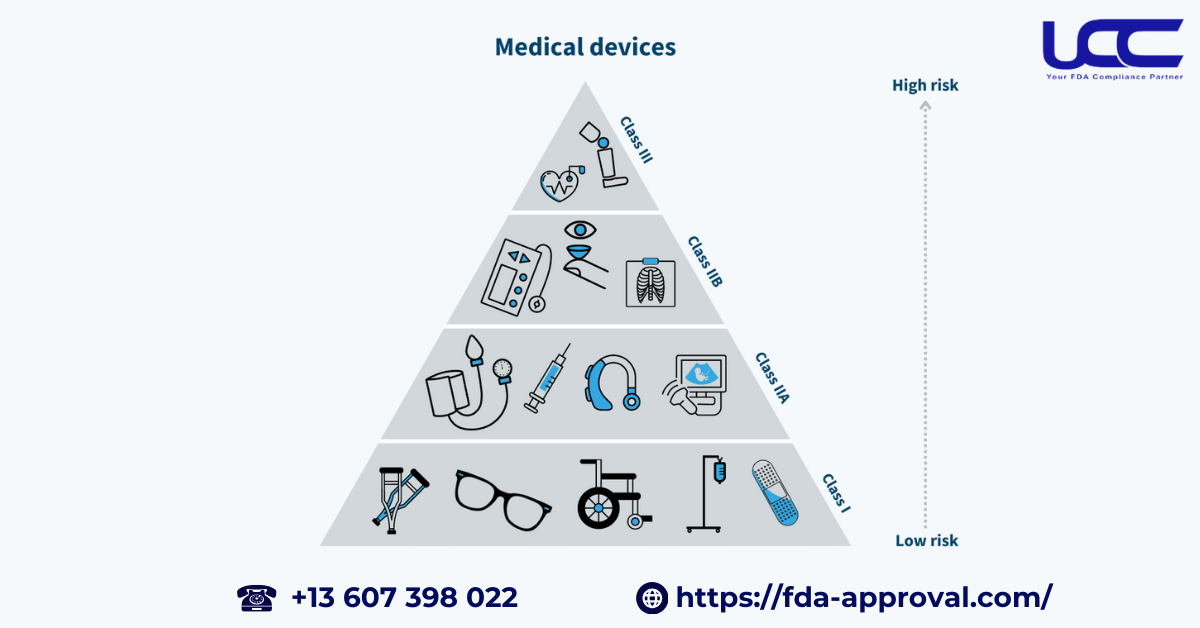
Before exploring the registration process, it’s important to familiarize yourself with the classification of medical devices. This understanding is key, as it dictates the requirements for FDA certification. The FDA categorizes medical devices using a three-tier system, which reflects different levels of risk:
Class I: General Controls
Devices classified as Class I, in fact, pose the lowest risk to users. Consequently, they must adhere to general controls and can enter the U.S. market by complying with FDA guidelines. Moreover, by following these established regulations, manufacturers can ensure the safety and efficacy of their products while facilitating a smoother market entry.
Examples include: bandages, cotton swabs, single-use medical instruments.
Class II: Special Controls
Class II devices present moderate to high risks. They require premarket notification and review before entering the market.
Examples include: blood pressure monitors, glucose meters, hearing aids.
Class III: Premarket Approval
Class III devices, in contrast, carry the highest risk and, therefore, necessitate prior approval before being marketed. Additionally, this stringent requirement underscores the importance of thorough evaluation to ensure the safety and effectiveness of these high-risk products. Furthermore, obtaining such approval is crucial for manufacturers seeking to establish credibility and trust in the marketplace.
Examples include: life-supporting devices, pacemakers, HIV diagnostic tests.
4. The Process to Register a Medical Device with the FDA
In this section, we will explore the steps involved in the FDA registration process. Specifically, we will outline each stage, emphasizing key requirements and considerations. Moreover, understanding these steps is crucial for ensuring compliance and facilitating a successful registration.

Step 1: FDA Requires Collecting Registration Information
You must collect specific information for FDA registration, including:
- The name and address of the facility, including full English name and abbreviation.
- Contact information for the head of the business.
- The type of business operations (manufacturing, processing, packaging, labeling, storage, etc.).
- A list of products manufactured or processed at the facility.
Step 2: Product Classification
Classification is essential to determine the requirements for FDA certification of medical devices.
Step 3: Choose a U.S. Agent
A U.S. Agent is, indeed, required for registering a medical device facility with the FDA. This representative serves as a vital communication link between your business and the FDA. Therefore, it is imperative to select a knowledgeable U.S. Agent who can effectively convey FDA communications to your organization. Furthermore, choosing an agent with extensive expertise will ensure that your business remains compliant with regulatory requirements and is well-informed about any updates from the FDA.
Step 4: Register DUNS Number
The DUNS number is a unique identifier for a business entity, recognized globally. This number is the only business identifier accepted by the FDA. Ensure that the DUNS information is accurate before proceeding with the registration.
Step 5: FDA Medical Device Registration
Step 6: Register and List Products
Manufacturers must register and list the devices they produce to help the FDA monitor and manage these products. Key components include:
- You must register all manufactured devices with the FDA.
- Assigning a UDI: Each device must have a unique device identifier according to the FDA classification system.
- Required Information for FDA Product Listing:
- Product name
- Instructions for use
- Manufacturer information
- Additional details as mandated
Step 7: Receive FDA Certification and Documentation
The FDA will review your registration application and may request additional information. Upon approval, you will receive a registration notice from the FDA.
5. Important Considerations When You Register a Medical Device with the FDA
- Registration Fees
There are, in fact, no exemptions or discounts available for facilities engaged in the manufacturing of medical devices. Accordingly, they have established the registration fee at $9,280 for the fiscal year 2025. Furthermore, it is important to emphasize that this fee applies universally, irrespective of the facility’s size or production volume.

- Annual Medical Device Registration Requirement
You must submit registration information annually between October 1 and December 31, even if no changes occur.
We hope this article has provided you with a clearer understanding of the process to register a medical device with the FDA. If you have any questions, please feel free to contact UCC for guidance and support. The UCC team looks forward to the opportunity to collaborate with you in the future!



