Are you aware that there is a category of medical devices allowed to be marketed in the United States despite not meeting all of FDA’s stringent testing requirements? In fact, this exception is called the Humanitarian Device Exemption (HDE). In this article, UCC will provide essential information regarding this special exemption.

1. Overview of Humanitarian Device Exemption (HDE)
Under the Orphan Drug Act (ODA) of 1983, rare diseases are defined as those affecting fewer than 200,000 individuals in the United States. Currently, of the 7,000 known rare diseases, only a small proportion have FDA-approved treatments. Besides, the primary challenge is that rare diseases affect a limited number of patients, making it difficult to gather sufficient evidence to meet FDA standards for safety and efficacy.

In response to this challenge, Congress introduced a provision in the Safe Medical Devices Act of 1990, which established a new regulatory pathway for devices intended for rare diseases affecting small populations. This provision, therefore, led to the creation of the Humanitarian Device Exemption (HDE).
The HDE allows manufacturers to market medical devices intended for rare diseases without needing full proof of effectiveness, provided they meet the necessary safety standards. Consequently, this pathway facilitates quicker access to critical devices for small patient populations.
2. What is a FDA Humanitarian Use Device (HUD)?
A Humanitarian Use Device (HUD) is designed to treat or diagnose a disease affecting fewer than 8,000 people annually in the U.S. (21st Century Cures Act, Pub. L. No. 114-255).
In simple terms:
- HUD is a device for humanitarian purposes.
- HDE is the special exemption that allows marketing without full effectiveness proof, but it ensures safety.

3. The Importance of Humanitarian Device Exemption
The HDE program plays a critical role in helping devices intended for patients with rare diseases. Here’s why:
- Ensures Access: The HDE lets devices be approved and marketed faster, helping patients with rare diseases access new treatments.
- Ensures Safety: The device must meet strict safety standards. Its benefits must outweigh risks compared to existing alternatives.
- Encourages Innovation: The HDE reduces barriers for developers, motivating them to create solutions for underserved patient communities.
4. Process for Obtaining HDE
4.1. Requirements for Devices Under HDE
The FDA can grant an Humanitarian Device Exemption if the device meets the following criteria:
- The device must not cause unreasonable risk of injury or illness. The benefits must exceed the risks.
- Without the HDE, the device wouldn’t be available to patients. No comparable device exists (except for those approved under HDE or IDE).
- The device must treat or diagnose a disease affecting fewer than 8,000 people annually in the U.S.
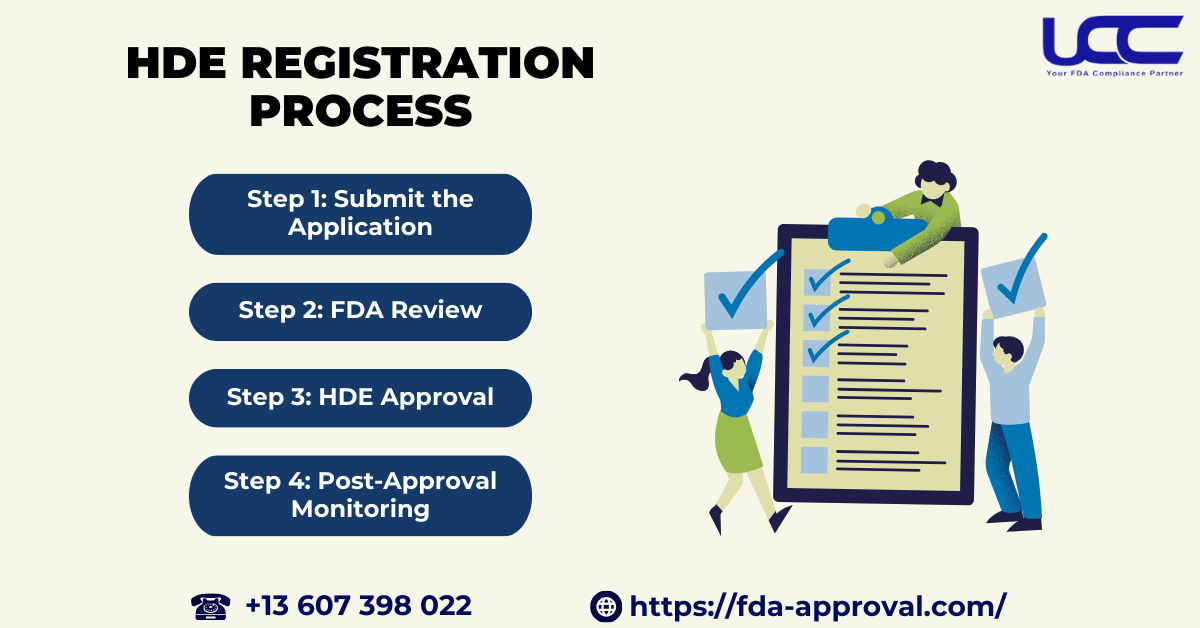
4.2. Registration Process
Step 1: Submit the Application
Manufacturers must submit an Humanitarian Device Exemption application to the FDA. Specifically, this includes proof that the device addresses a rare disease, has no alternatives, and is safe.
Step 2: FDA Review
The FDA will review the application based on safety and intended use. If the device meets safety standards, the FDA will confirm no equivalent device exists. This review is faster than traditional approval processes like PMA or 510(k).
Step 3: Humanitarian Device Exemption Approval
Once the FDA confirms the device meets all criteria, they will approve the HDE. Manufacturers can produce and use the device in approved healthcare settings. However, they won’t market it as widely as devices cleared through PMA or 510(k).
Step 4: Monitoring After HDE Approval
After approval, the FDA will monitor the device’s use to ensure safety. Manufacturers must report any safety issues during use.
5. Conclusion
The Humanitarian Device Exemption (HDE) is crucial for providing medical devices to treat rare conditions. Moreover, by simplifying the approval process for Humanitarian Use Devices (HUDs), HDE ensures patients with rare diseases get access to life-saving treatments faster. While manufacturers don’t need to provide full proof of effectiveness, they must prioritize safety above all.
If you’re involved in the development or use of rare disease treatments, understanding the HDE pathway is key. UCC is here to assist with any FDA-related questions and guidance on HDE.



