Do you consider labeling medical devices in accordance with FDA standards to be the most challenging process? Certainly not. In this article, UCC will provide you with FDA guidance labeling medical devices to help you gain a clearer understanding of the general regulations for labeling medical devices set by the FDA.
1. The Importance of Medical Device Labels
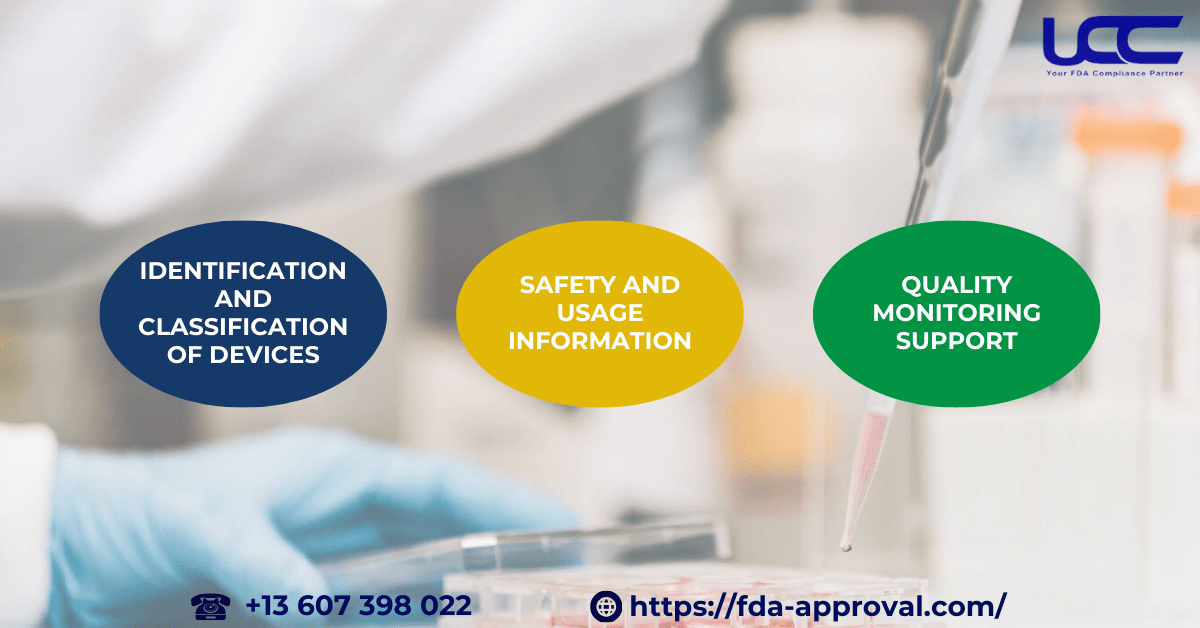
The FDA guidance on labeling medical devices covers various regulations related to labeling. In addition, here are some key components of medical device labels as per FDA standards:
-
Identification and Classification of Devices
Medical device labels provide basic information to identify the device, including its name, intended use, and essential technical specifications. As a result, this helps users distinguish between devices and understand their functions.
-
Safety and Usage Information
Medical device labels must provide clear instructions for safe use, including warnings, storage guidelines, maintenance instructions, and potential risks. Therefore, proper labeling helps prevent accidents and malfunctions during use.
-
Quality Monitoring Support
Additionally, medical device labels are essential in monitoring the quality and compliance of the device as per 21 CFR Part 820, the Quality Management System (QMS) regulations. In turn, correctly monitoring the label helps verify that the device maintains quality throughout its lifecycle.
Therefore, the label does not just protect the user but also ensures legal compliance and quality management across the entire process—from production to usage.
2. General FDA Guidance Labeling Medical Devices
The FDA guidance labeling medical devices covers various regulations related to labeling. In particular, here are some key components of medical device labels as per FDA standards:
2.1. Name and Business Location
The manufacturer, packer, or distributor must include their name and address on the label. They should clearly list the street, city, state, and ZIP code. If the company’s address is listed in the local telephone directory, they may not need to include it on the label.
2.2. Intended Use of the Device
When a distributor, packer, or seller intends to use the device for a purpose other than its original intended use, they must update the label to reflect the new purpose. For instance, if a distributor sells a dental X-ray device to podiatrists, they must update the label to indicate its new usage.
If the manufacturer knows or has information that the device will be used for purposes other than what was originally intended, the label must be updated accordingly. Consequently, this ensures that all parties comply with FDA guidance labeling medical devices.
2.3. Adequate Directions for Use
The term “adequate directions for use” refers to instructions that allow an average person to safely and effectively use the device for its intended purposes. Specifically, the full directions should include:
- Intended Use and Conditions: The manufacturer should state what the device is used for and under what conditions on the label.
- Dosage Information: Provide dosage details for different age groups or physical conditions.
- Frequency of Use: Indicate how often the device should be used within a given time frame.
- Duration of Use: Specify the time duration for using the device.
- Time-related Factors: Indicate how the timing of usage may vary depending on factors like meals or time of day.
- Method of Application: The manufacturer must detail how the device should be applied on the label.
- Preparation: If preparation is needed before use, the label should include these steps.
2.4. FDA Medical Device Color Additive Information
FDA labeling requirements for medical device color additives are essential for ensuring safety and compliance. Specifically, all color additives used in medical devices must be clearly labeled with detailed information, including the name of the additive, any usage restrictions, and the permissible limits for specific applications. This not only ensures that manufacturers adhere to FDA regulations, but also guarantees that consumers can confidently use the products. Furthermore, proper labeling allows for traceability, which helps to verify compliance with safety standards and facilitates any necessary recalls or investigations.
3. Additional Notes of FDA Guidance Labeling Medical Devices
3.1. Incorrect Labeling According to FDA Guidance on Labeling Medical Devices
The manufacturer labels a device incorrectly if it makes false or misleading claims about the device, drug, food, or cosmetic.
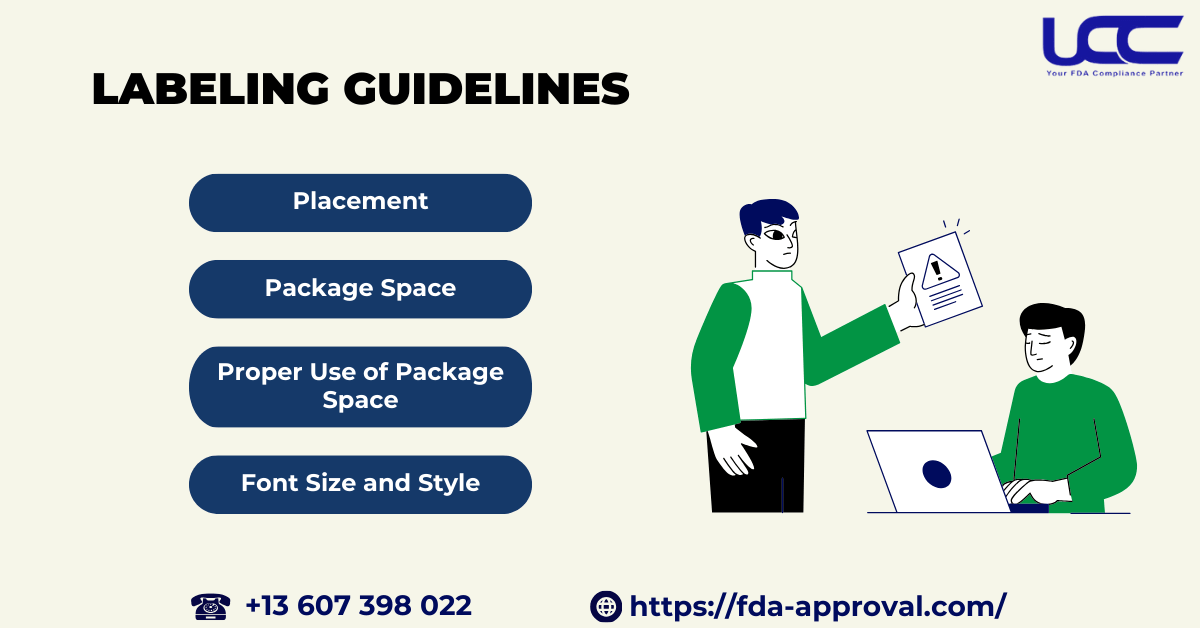
3.2. Labeling Guidelines
Here are some important notes from the FDA guidance labeling medical devices:
- Placement: Required information must appear on the parts of the package that are visible to the consumer under normal conditions of purchase.
- Package Space: If the manufacturer provides sufficient space but fails to show the required information on at least two sides of the package, they violate the guidelines.
- Proper Use of Package Space: The manufacturer should fully utilize the available space on the label and avoid obstructing it with non-required information or excessive labeling.
- Font Size and Style: The font should be appropriately sized and have a contrasting color to the package background to ensure readability. Furthermore, avoid designs that obscure the label.
4. Use of Symbols on According to FDA Guidance on Labeling Medical Devices

The FDA guidance on labeling medical devices includes specific rules regarding the use of symbols. In particular, the FDA issued a final rule on June 15, 2016, which became effective on September 13, 2016. This rule permits the use of certain symbols on medical device labels. As a result, manufacturers now have three options for incorporating these symbols in their labeling:
- Do Not Use Symbols: Manufacturers may choose not to use symbols.
- Symbols with Adjacent Text: Manufacturers may use symbols alongside explanatory text.
- Independent Symbols: Manufacturers may use symbols independently, as long as they meet specific criteria. Moreover, manufacturers must include these symbols in a glossary of symbols on the device label.
Manufacturers should replace small, hard-to-read text with symbols to make labels more user-friendly. This step is especially crucial in medical device labeling, where space often gets limited.
In conclusion, understanding FDA guidance for labeling medical devices is essential for compliance and safety. By following these detailed guidelines, manufacturers can effectively label their devices to meet regulatory requirements while enhancing usability and user understanding. And finally, don’t forget to reach out to UCC if you need support with any FDA-related issues!
For more information: Medical Device Facility Registration Service



