Are you engaged in the cosmetics business and seeking to enter the U.S. market? This article has been specifically prepared for your benefit. In this discussion, UCC will furnish you with vital information concerning the labeling requirements for cosmetic products as mandated by the FDA. The following sections will encompass all relevant definitions, requirements, and procedures related to FDA cosmetic product labels.

1. Definition of Cosmetics According to the FD&C Act
Cosmetics are items applied to the human body for the purpose of cleansing, beautifying, enhancing attractiveness, or altering appearance without affecting the structure or function of the body. Consequently, cosmetics include products such as moisturizers, perfumes, lipsticks, nail polishes, eye and face makeup, shampoos, permanent wave solutions, hair dyes, toothpaste, deodorants, and any materials used as ingredients in cosmetic products.
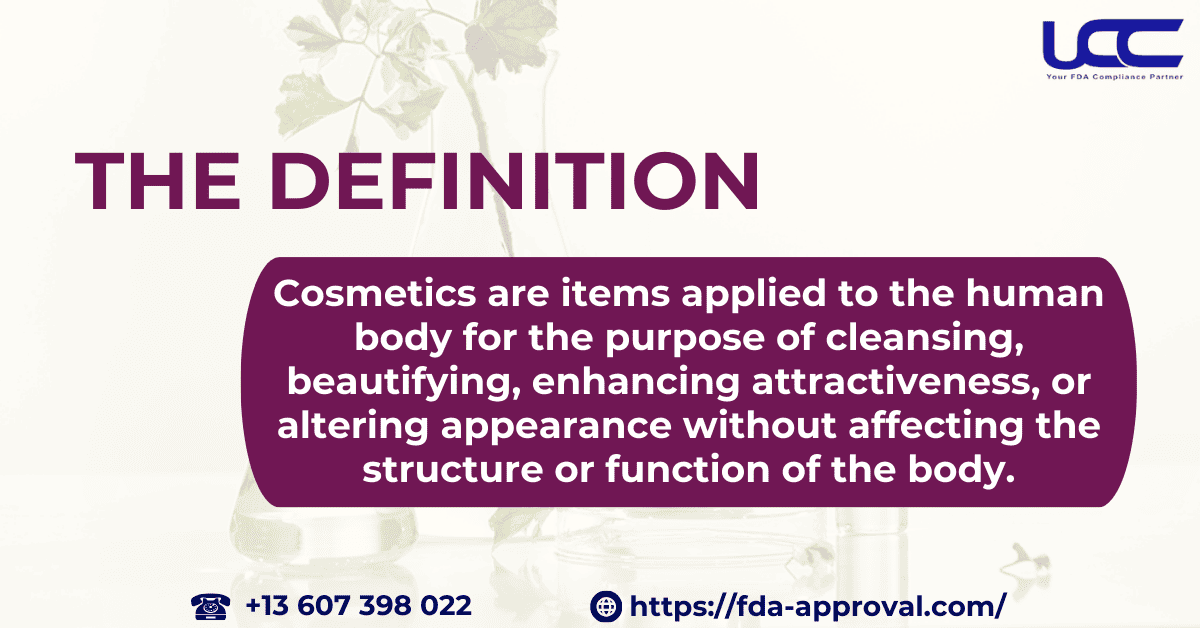
Furthermore, products intended for treatment or prevention of disease, or that affect the structure or function of the human body, will be classified as drugs. Such products must comply with both drug and cosmetic regulations.
2. General Requirements for Cosmetic Product Labels
2.1. Principal Display Panel (PDP)
- Product Name on Cosmetic Product Labels
The name of the product must be clear and easily recognizable. This may include the brand name, specific product name, or both.
- Weight
The actual weight or volume of the product must be indicated. This should be expressed in appropriate units of measurement for the target market.
- Ingredients
All ingredients must be presented in descending order based on their weight. Besides, the names of the ingredients should use scientific nomenclature or common names accepted by the FDA.
- Warning Labels
Appropriate warning labels must be included to ensure consumer safety. For instance, examples include: “For external use only,” “Keep out of reach of children,” and “May cause skin irritation.”

2.1. Information on the Information Panel (IP) for Labeling
- Directions for Use
Provide complete information on how to use the product safely and effectively. Moreover, ensure that users understand any precautions they should take during use.
- Manufacturer or Distributor Name and Address on Labeling
This information allows consumers to contact the company if necessary.
- Information on Active Ingredients and Product Benefits
This information helps consumers better understand the function and effects of the product.
- Batch Number or Production Code of cosmetic product
The batch number or production code helps the manufacturer track the product and implement recall measures if necessary.
- Expiration Date
The expiration date shows the period during which consumers can use the product safely and effectively.
- Any additional essential information that may be required.
3. Regulations on Specific Ingredients for Cosmetic Product Labels
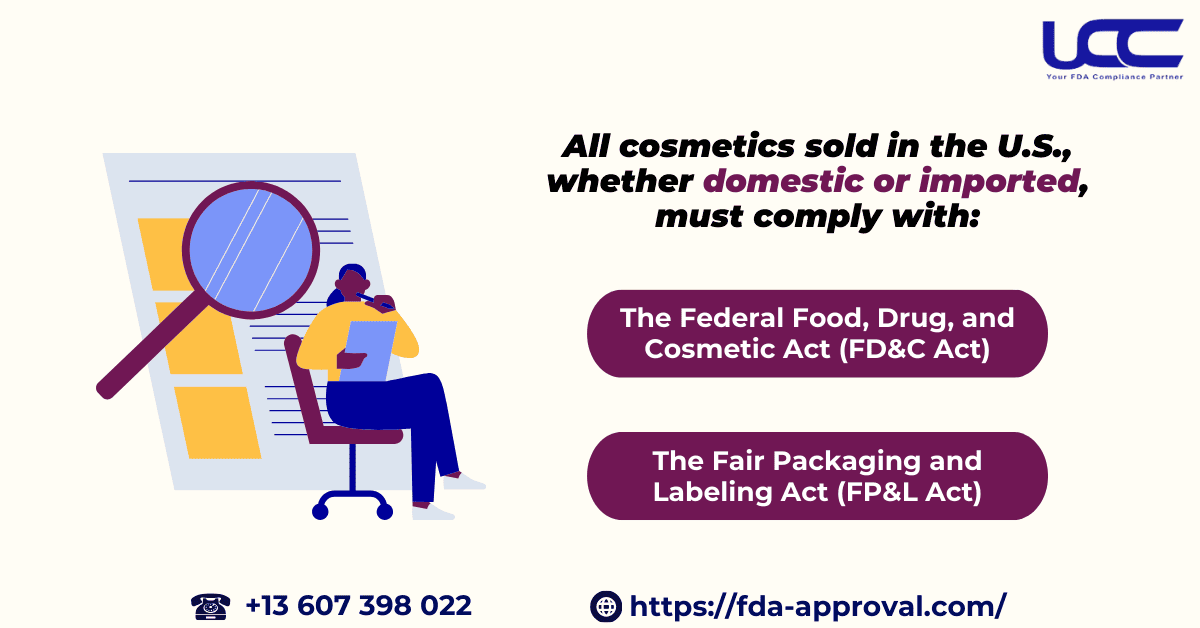
All cosmetics sold in the United States, whether produced domestically or imported, must adhere to the provisions of:
- The Federal Food, Drug, and Cosmetic Act (FD&C Act)
- The Fair Packaging and Labeling Act (FP&L Act)
3.1. Declaration of Ingredients
The ingredient declaration must be clearly visible and legible at the time of purchase. Additionally, this declaration can appear on any information panel of the packaging, such as fold-out boxes or labels. Companies can also display the information on stickers or tags affixed to the product, ensuring that customers can easily access ingredient information. Finally, they must list ingredients in descending order of predominance by weight.
3.2. FDA Cosmetic Color Additive
The FDA actively regulates color additives in cosmetics through strict approval processes outlined in U.S. law, specifically the FD&C Act. Furthermore, all color additives are documented in Title 21 of the Code of Federal Regulations (CFR).
3.3. FDA Fragrance Allergen Labeling Requirements

As mentioned earlier, an ingredient list is mandatory for cosmetic product labels. In most cases, companies must list all components, including fragrances, individually. However, U.S. regulations allow them to identify fragrance and flavor ingredients simply as ‘Fragrance’ or ‘Flavor’ for proprietary formulation reasons.
3.4. Cosmetic Product Labels Warnings
Certain cosmetics may pose risks to consumers if misused. Therefore, such products must feature appropriate warning labels and clear usage instructions. To ensure consumer safety, these statements should be prominent and easily noticeable. Additionally, the FDA mandates that some cosmetics must carry specific warnings.
3.5. Anti-Counterfeiting Measures
Packaging must include a statement prominently displayed to alert consumers to anti-counterfeiting features.

3.6. Cosmetic Product Labels Claims
Although there are no pre-market approval requirements for claims, the FDA enforces limits on cosmetic label claims. By law, the information on cosmetic product labels, including claims, must be truthful and not misleading. The FDA retains the authority to take action against companies that violate these labeling regulations.
4. Consequences of Incorrect Cosmetic Product Labels
A cosmetic product is considered improperly labeled if:
- The labeling is inaccurate or deceptive.
- The name and address of the manufacturer, packer, or distributor are missing
For improper labeling, the FDA has implemented several enforcement actions, including:
4.1. Warning Letters
The FDA can send warning letters to companies that make unapproved drug claims in cosmetic products or if they incorrectly label their products. Additionally, these actions can lead to further regulatory consequences.
4.2. Regulatory Action
The FDA can take regulatory action against companies and cosmetics with incorrect or misleading labels.
4.3. Legal Action
The FDA may pursue legal action against companies and individuals marketing improperly labeled or counterfeit cosmetics.

We hope this article has provided you with valuable insights into FDA cosmetic labeling requirements, highlighting the significance of proper labeling. If you are seeking a partner to assist you in entering the U.S. market, UCC offers comprehensive services, including FDA registration and cosmetic labeling consultation for businesses. Please contact UCC to benefit from our competitive pricing.



