A nutrient content claim refers to any statement on a food label that describes the level of a specific nutrient in the product. At UCC, we specialize in helping businesses navigate FDA regulations to ensure their claims meet all compliance standards. In this article, we will outline the definition, requirements, and considerations for using nutrient content claims in compliance with FDA guidelines.
1. What is a FDA Nutrient Content Claim?
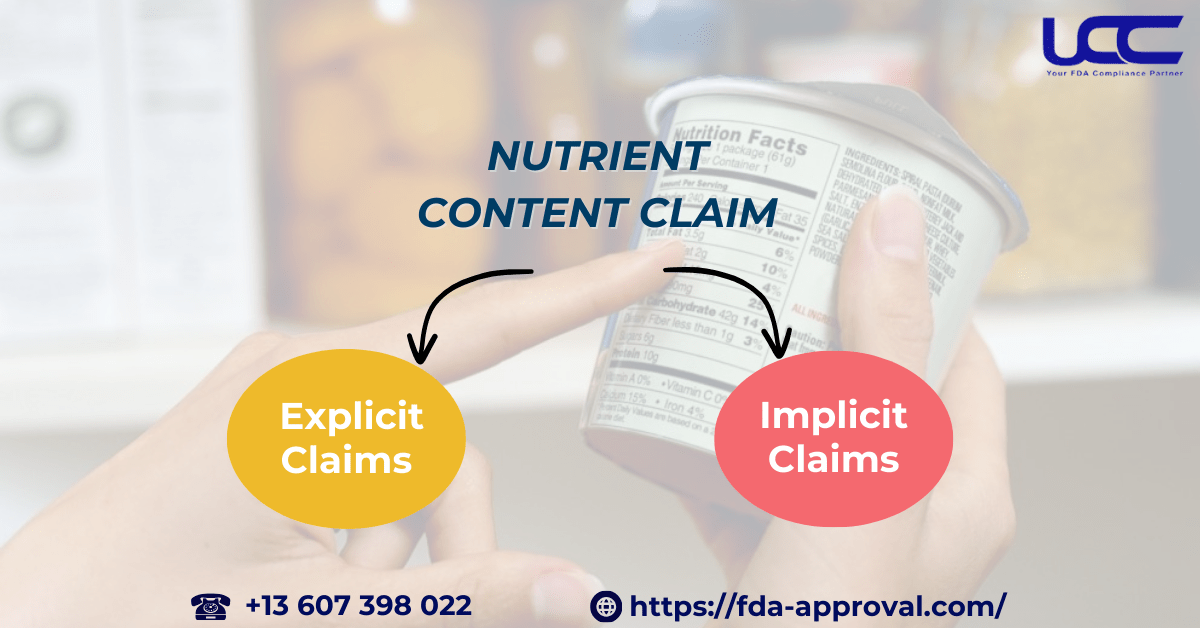
These claims are subject to strict regulations by the U.S. Food and Drug Administration (FDA) under Title 21 of the Code of Federal Regulations (CFR), Chapter I, Subchapter B. Specifically, a nutrient content claim explicitly or implicitly describes the amount of a nutrient in a food product.
1.1. Explicit Claims
Explicit claims directly state the nutrient’s level. For example:
- “Low sodium”
- “Contains 100 calories”
1.2. Implicit Claims
Implicit claims suggest the presence or absence of nutrients indirectly. For instance, these may include:
- Descriptions of Ingredients: Statements implying the presence of a specific nutrient, such as “rich in oat bran.”
- Health-Related Suggestions: Claims linking the food’s nutrient content to a healthy diet, such as “healthy, contains 3 grams of fat.”
The FDA allows these claims as part of a brand name only if approved beforehand.
2. FDA Requirements for Nutrient Content Claim
2.1. Fundamental Rules
To make a nutrient content claim, products must:
- Refer to nutrients with established Daily Values (DV).
- Meet FDA-defined nutrient criteria.
For Instance:
- “Low Fat”: The food must contain less than 3 grams of fat per serving.
- “Only 200 mg Sodium”: Such claims must meet the FDA’s “low sodium” standards or include a disclaimer like “not a low-sodium food.”
2.2. Specific Regulations for Nutrient Content Claim
a. Comparative Terms: “Less” “Fewer” and “More”
These terms compare the nutrient content of two foods. The reference food can be:
- A different but substitutable item (e.g., pretzels compared to potato chips).
- A similar product (e.g., one brand of multivitamins compared to another).
b. Additional Terms: “Light” “Reduced” “Fortified” and “Enriched”
These terms require the reference food to be a similar product:
- “Light” Claims: The reference food must represent typical items in the same category. The reference data should come from reliable sources, such as databases or averages of leading brands.
Examples:
- “Less Sodium” Snack: Compared to regular chips, both consumed as snacks.
- “Light” Cream Cheese: Compared to the average nutritional values of popular cream cheeses.
- “Fortified with Vitamin D” Milk: Compared to regular milk without added nutrients

3. Key Considerations for Using Nutrient Content Claim
3.1. Type Size and Style
- The claim’s font size must not exceed twice the size of the Statement of Identity (product name).
- The font style must not overshadow the product’s official name.

3.2. Placement and Disclaimer Requirements
- Disclaimers should appear near the claim on the label.
- The disclaimer text must be clear and at least as large as the required font size for net quantity statements.
Using a nutrient content claim effectively requires strict adherence to FDA rules. Furthermore, these claims are essential in providing consumers with clear information about a product’s nutritional value. Consequently, they promote transparency and support healthier consumer choices. However, to maintain consumer trust and avoid regulatory penalties, these claims must be accurate, compliant, and presented correctly.
For more information: Food Safety Modernization Act: Key Insights and Overview
By understanding these regulations, food manufacturers can navigate the labeling process with confidence while promoting their products’ nutritional benefits. In addition, compliance enhances consumer trust and distinguishes products in a competitive market. As a result, adhering to these guidelines ensures legal protection and upholds transparency with customers. Please contact UCC if you need assistance with FDA compliance and product labeling.



