Concerns regarding food safety have been growing. As a result, the FDA’s Food Safety Modernization Act (FSMA) introduces stringent regulations. These regulations are designed to enhance food traceability. In this article, UCC will offer valuable insights into the food traceability rule.
1. Overview of the Food Traceability Rule
The FDA’s FSMA mandates that food manufacturers retain additional traceability records for specific food items, improving food tracking and traceability, especially during foodborne illness outbreaks or contamination incidents. To facilitate this, the FDA has published a Food Traceability List (FTL), which includes foods such as leafy greens, melons, cut fruits, and certain seafood.
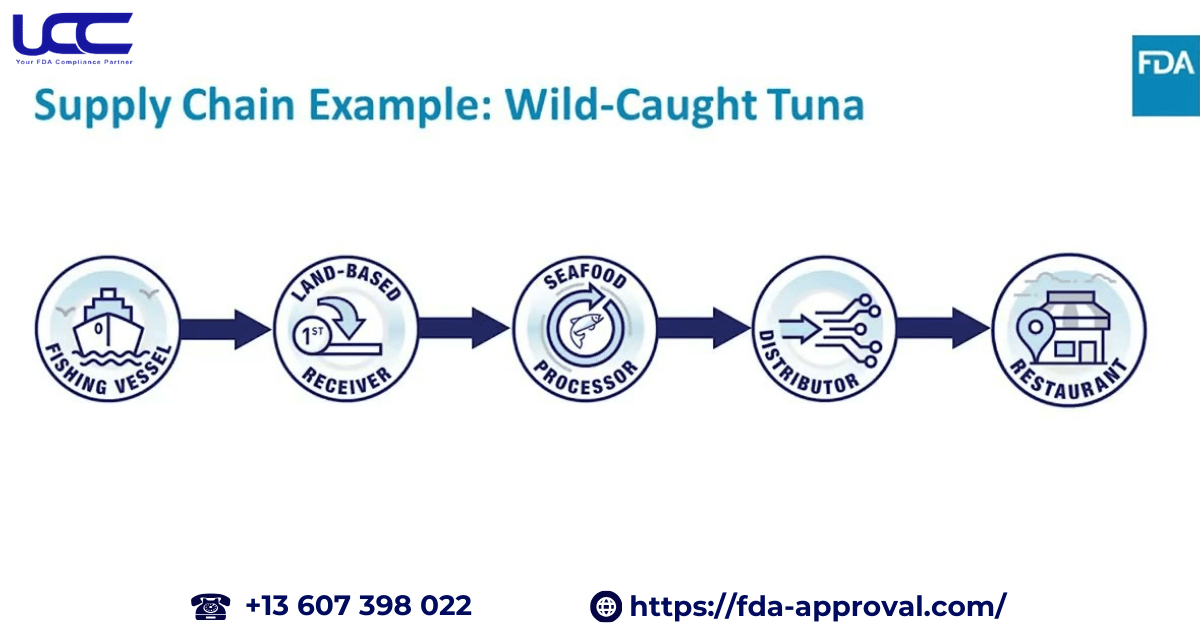
Furthermore, in 2022, the FDA issued the final Food Traceability Rule, requiring businesses handling these foods to keep extra traceability records. This regulation is a key component of the Smarter Food Safety Plan, established under Section 204(d) of FSMA. These records also enable businesses to track food movement through the supply chain, speed up responses during contamination events, and facilitate product recalls to protect public health.
2. Role of the Food Traceability Rule
As mentioned earlier, the Additional Traceability Records for Certain Foods play a vital role in ensuring food safety in the U.S. market. Specifically, it delivers several key benefits:
- Quick Identification of Sources: The rule enables rapid identification of contaminated food sources during outbreaks, aiding in targeted responses.
- Efficient Recalls: It supports precise product recalls, minimizing consumer exposure to hazardous products.
- Risk Management: By helping businesses identify and prevent risks proactively, it reduces the potential for harm.
- Regulatory Compliance: The rule ensures that businesses comply with FDA safety standards. Additionally, it provides essential documentation for audits and investigations.
- Public Health Protection: Strengthening food safety reduces foodborne illnesses and fosters consumer trust in food systems.

3. Types of Foods Covered Under the Food Traceability Rule
The regulation mandates that individuals and entities engaged in the production, processing, packaging, or storage of foods listed in the FTL must, therefore, retain records containing critical data. Furthermore, these records must be made available to the FDA within 24 hours, or within a timeframe reasonably agreed upon. The rule aims to enhance food traceability in the supply chain and enable rapid responses in case of food safety incidents.
The Food Traceability List (FTL) identifies specific foods that require additional traceability records, including products containing ingredients from these foods, as long as the ingredient maintains its original form (e.g., fresh). Some examples include:
- Cheese: Soft cheeses, soft-ripened cheeses, and unpasteurized cheeses (excluding hard cheeses).
- Shell Eggs: Eggs with shells.
- Nut Butters: Nut butters made from tree nuts and peanuts (excluding soy nut butter).
- Leafy Greens (fresh or cut): All types of leafy greens like lettuce and kale.
- Fresh Melons and Tropical Fruits: Melons, mangoes, papayas, etc.
- Finfish: Fish prone to histamine production or contamination with ciguatoxin, among others.
- Crustaceans: Including shrimp and crab.
- Deli Salads: Ready-to-eat salads such as potato salad, egg salad, etc.
For more information: Food Label Consultants: Everything You Need to Know
4. Key Considerations for Compliance
4.1 Timeline
Routine inspections under the Food Traceability Rule will begin in 2027. However, the deadline for compliance with the regulation is set for January 20, 2026. Therefore, this timeline provides stakeholders with ample opportunity to cooperate and ensure the proper maintenance and sharing of traceability information within the supply chain.
4.2 Methodology
Under FSMA Section 204(d)(1)(C), the final rule does not mandate specific technologies for record storage. However, records may be paper-based or accurate copies of original electronic records, such as scanned images or digital replicas. Furthermore, these records must be legible, clear, and stored in a manner that prevents damage or loss. In addition, electronic records can include valid and active electronic links to the required information.
4.3 Penalties for Non-Compliance
- Voluntary Corrective Actions: The FDA will issue reminder letters (untitled letters and warning letters) to companies requesting voluntary corrective actions before enforcement actions are taken.
- Civil Actions: If voluntary compliance is not achieved, the federal government may pursue civil actions in federal court to enforce compliance.
- Criminal Actions: In severe cases, companies may face criminal prosecution in federal court.
- Import Refusals: Food products not meeting the record-keeping requirements may be denied entry under Section 801(a)(4) of the FD&C Act.

In conclusion, the FDA’s food traceability regulations not only promote transparency in the food supply chain but also aid businesses in addressing food safety incidents effectively. Moreover, should your business have any questions or require assistance with FDA compliance, UCC is available to provide expert guidance and support. Our team of experts is well-versed in FDA regulations and is ready to support businesses in ensuring compliance, expanding market opportunities, and protecting their brand.



