In this article, UCC provides crucial information about the FDA’s Premarket Approval (PMA) process for medical devices. This essential process ensures that high-risk medical devices meet strict safety and efficacy standards before being launched in the U.S. market. Join UCC as we explore the requirements, procedures, and key considerations to help businesses navigate the FDA medical device premarket approval process successfully.

1. What is FDA Medical Device Premarket Approval?
1.1. The definition of FDA Medical Device Premarket Approval
Similar to the 510(k) process, Premarket Approval (PMA) is a scientific evaluation process that assesses the safety and effectiveness of Class III medical devices. Specifically, Class III devices are those that sustain or support life, are critical in preventing health deterioration, or carry substantial risks if used improperly. Consequently, the FDA mandates PMA as the most stringent approval process. Manufacturers must receive FDA approval for their PMA application before marketing their devices.
1.2. Who Needs to Apply for PMA?
The PMA requirements apply to Class III devices, which represent the highest risk category in medical device regulation. If businesses are unsure whether their device requires PMA, they can search the product code in the FDA’s Pre-market Approval (PMA) and Premarket Notification (510(k)) databases.
2. Methods for Submitting a FDA Medical Device Premarket Approval
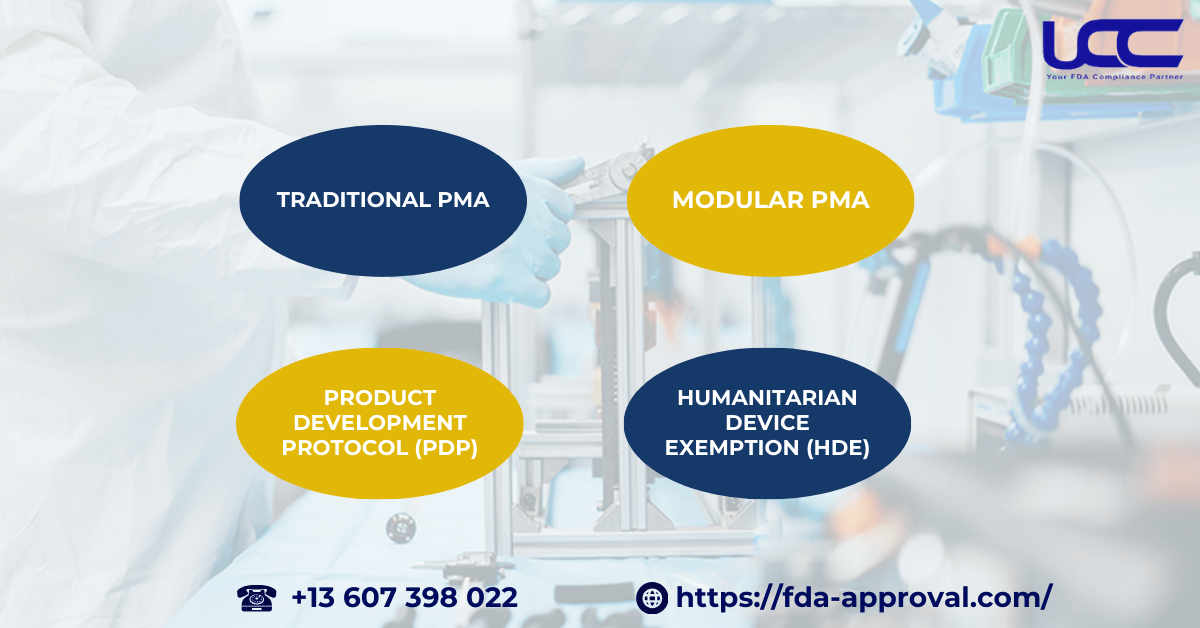
There are four main methods available for submitting a FDA Medical Device Premarket Approval application:
2.1. Traditional PMA
Manufacturers most commonly use the traditional PMA method. This approach requires submitting a complete PMA application as a single package. Manufacturers typically use this method when clinical trials are complete. The application must include comprehensive scientific and regulatory assessments, including device descriptions, intended use, clinical and non-clinical research data, and other documentation.
2.2. Modular PMA
The modular PMA method is used for medical devices still in the early stages of clinical trials. This method allows manufacturers to submit the PMA in distinct modules, each addressing specific aspects of the device and its required documentation. The FDA reviews each module as it is submitted, helping to reduce the review time and cost compared to a full PMA submission. This method is ideal for complex devices requiring extensive testing and data analysis. However, it is not suitable when the device design is near completion or subject to change.
2.3. FDA Product Development Protocol (PDP)
The Product Development Protocol (PDP) method is intended for medical devices in the early stages of development as well as for technologies that are already well-established. This method, therefore, facilitates collaboration between the manufacturer and the FDA, optimizing both the development and clinical testing phases. As a result, manufacturers can receive feedback throughout the process, ensuring their device meets the FDA’s regulatory requirements from the outset.
2.4. FDA Humanitarian Device Exemption (HDE)
The FDA offers the Humanitarian Device Exemption (HDE) process for medical devices that treat or diagnose diseases or conditions affecting fewer than 8,000 people annually in the U.S. This process is less stringent than the FDA’s Medical Device Premarket Approval, and it does not require clinical evidence of effectiveness, although manufacturers must demonstrate safety. If FDA approves the device, manufacturers can market it for diagnosing or treating rare diseases, as long as it meets specific use and profit limitations.
3. The FDA Medical Device Premarket Approval Submission Process
3.1. Required Elements of the PMA Application
The PMA application must include the following components:
- A report detailing all known safety and efficacy information about the device.
- A description of the device, including its components, features, and operational principles.
- A description of the manufacturing and processing methods for the medical device.
- Results from non-clinical studies, including laboratory or animal testing.
- The outcomes of any clinical trials that have been conducted.
- Proposed labeling for both professional and patient use.
- A summary of the data pertaining to the safety and efficacy of the device
3.2. The PMA Approval Process
The FDA conducts multiple stages in the PMA process for medical devices to ensure that a device is safe and effective before it can be marketed.
- Initial Review: FDA examines the application for completeness and organization. This stage may require up to 45 days.
- Substantive Review: The FDA performs an in-depth evaluation to verify adherence to scientific, regulatory, and quality standards. This phase can take as long as 180 days.
- Advisory Panel Review: In some cases, the FDA may convene an advisory panel of experts to review the application.
- Decision Notification: If there are no reasons for denial, the FDA will approve the application publicly.
The FDA may also perform a pre-approval inspection to ensure that the manufacturer can design and produce the device as described in the application.

3.3. FDA Medical Device Premarket Approval Review Timeframe
The FDA Medical Device Premarket Approval process typically takes between 6 and 36 months before approval. Additional clinical trials may extend the timeline, often resulting in several years before approval is granted for a product. While the typical review time for a PMA application is 6 months, it is often longer. During this period, manufacturers must provide valid scientific evidence demonstrating the device’s safety and efficacy.
4. UCC: Your Trusted Partner in FDA PMA Approval
assists businesses across various sectors, including pharmaceuticals, medical devices, food, and cosmetics. Moreover, our expert team is well-versed in FDA regulations, enabling us to help clients efficiently prepare and submit their PMA applications.

UCC supports businesses throughout the entire FDA approval process with a focus on quality and client satisfaction. We help companies save time and costs while ensuring their products meet all necessary safety and efficacy standards before entering the U.S. market.
We hope this article has provided you with valuable insights into the FDA’s Premarket Approval (PMA) process for medical devices. If your company is seeking a professional and reliable partner to assist with FDA approval, don’t hesitate to contact UCC today!



