As you may be aware, the FDA regulates the majority of packaged foods sold in the United States, with specific requirements for the information that must be included on food labels. These regulations have a direct impact on the marketability of products within the industry. Accordingly, in this article, UCC is pleased to provide valuable insights regarding Food Label Consultants and FDA Food Nutrition Labeling.

1. Concept Of FDA Food Label Consultants
1.1. Definition of labeling
In line with the Federal Food, Drug, and Cosmetic Act (FFDCA):
Section 201(m) defines ‘label’ as a display of written, printed, or graphic matter upon any article or the immediate container (not including packaged liners) of any article; and the term “labeling” means all labels and other written, printed, or graphic matter (1) upon any article or any of its containers or wrappers, or (2) accompanying such article.

1.2. The process of changing food labels by the FDA
In recent years, there have been significant changes to food labels. Specifically, in the spring of 2016, the U.S. Food and Drug Administration unveiled a new food label design that reflects current evidence-based dietary recommendations. This design highlights the connection between diet and chronic diseases such as obesity and heart disease. As a result, this initiative aims to provide consumers with information and education to enhance their understanding of the foods they consume and how these foods can impact their health. Moreover, the latest version of the food label was mandated for implementation on January 1, 2020, for larger food manufacturers, while smaller producers were given an extension until January 1, 2021, to adopt the new labeling requirements.
The updated food labeling regulations provide significant benefits that enhance consumer awareness and public health. By offering clear and accessible nutritional information, these labels empower individuals to make informed dietary choices, potentially reducing the incidence of chronic diseases such as obesity and heart disease. Additionally, the focus on ingredient transparency fosters a better understanding of food products, enabling consumers to evaluate added sugars and artificial components. These reforms not only ensure legal compliance among manufacturers but also promote healthier options and encourage innovation in the food industry, ultimately contributing to the overall well-being of the population.
1.3. Role of food labels
Labels play a vital role for both consumers and producers, especially in accordance with the Food Label Consultants. They enhance food safety and safeguard public health. Furthermore, these labels support effective business practices by fostering consumer trust and regulatory compliance.
- For Consumers:
Labels play a crucial role in providing accurate product information. They help consumers understand the ingredients and nutritional content. Additionally, labels indicate where products come from and their shelf life. They also offer usage instructions and storage guidelines. Furthermore, labels include warnings about potential risks, such as allergens. This allows consumers to make informed choices and compare prices effectively. Consequently, consumers can evaluate the quality of different products, leading to more economical purchasing decisions.
- For Producers:
Conversely, well-designed packaging boosts brand reputation. It ensures that information is complete and accurate, fostering trust in both the product and the brand. Proper labeling also guarantees compliance with legal standards, helping to avoid potential legal issues and safeguard interests. Adhering to regulations showcases professionalism, ultimately helping products stand out and attract customers.
2. FDA Food Labeling Regulations
In this section, let us delve deeper into the specific regulations, as advised by food label consultants . We will explore these requirements in detail to ensure a comprehensive understanding.

2.1. General regulations
2.1.1. Required Information on Labels
According to the regulations set by the Food and Drug Administration (FDA), food producers are required to include the following information on their product labels:
- Common Name or Brand Name of the Product: This is the name by which consumers identify the product. You must prominently display it on the Principal Display Panel (PDP), which is the front face of the product.
- Identification Standards
- Ingredient List
- Nutrition Facts Table: Typically found on the back, this table includes detailed information about nutrients, vitamins, minerals, and macronutrients. Not every product is required to have this label, but it is important to check for exemptions.
- Allergen Information
- Health Claims
- Food Safety Warnings
- Industry and Address of theManufacturer or Importer
- Origin of the Product
- Expiration Date
- Usage and Storage Instructions
- Serving Size Recommendations
- Actual Contents
2.1.2. Format and Presentation of Information on Food Labels
Food Label Consultants outline several important requirements regarding the format of food labels, including the following:
- Layout

- Size and Font
Food labels should feature a font that is large enough for consumers to read easily. Specifically, the FDA recommends that producers use a font size of at least 1/16 inch (approximately 1.6 mm) for crucial information such as the product name, ingredient list, and nutrition facts.
Moreover, producers should ensure the font is clear and legible, which helps consumers differentiate between characters and words. Additionally, using a combination of uppercase and lowercase letters effectively highlights and emphasizes important information.
- Color
Producers must use colors on food labels in a harmonious and visually appealing manner. Furthermore, they should choose contrasting colors to clearly distinguish between the information and the label background.
In addition, they should avoid using colors that mislead or distort the perception of the product’s quality or ingredients.
- Language
Producers must write information on food labels in English, while also allowing for additional languages. If they present information in a language other than English, they must provide an accurate and complete English translation to ensure clarity.
- Images and Illustrations
The FDA allows producers to use images and illustrations on packaging to clarify information or attract consumer attention. However, producers should ensure that images and illustrations are relevant to the product’s content and do not mislead consumers.
Additionally, they should select images that are of high quality and easy to see, thereby ensuring these visuals effectively support the label’s information.
2.2. Specific regulations for FDA food labeling
2.2.1. FDA Food Nutrition Labeling
FDA Food Nutrition labeling, as advised by food label consultants, play a vital role in providing essential information about the food that consumers consume. Additionally, the FDA regulates the necessary label formats for your products based on the size and content of the packaging. Food manufacturers are required to include the following information on FDA Food Nutrition Labeling:
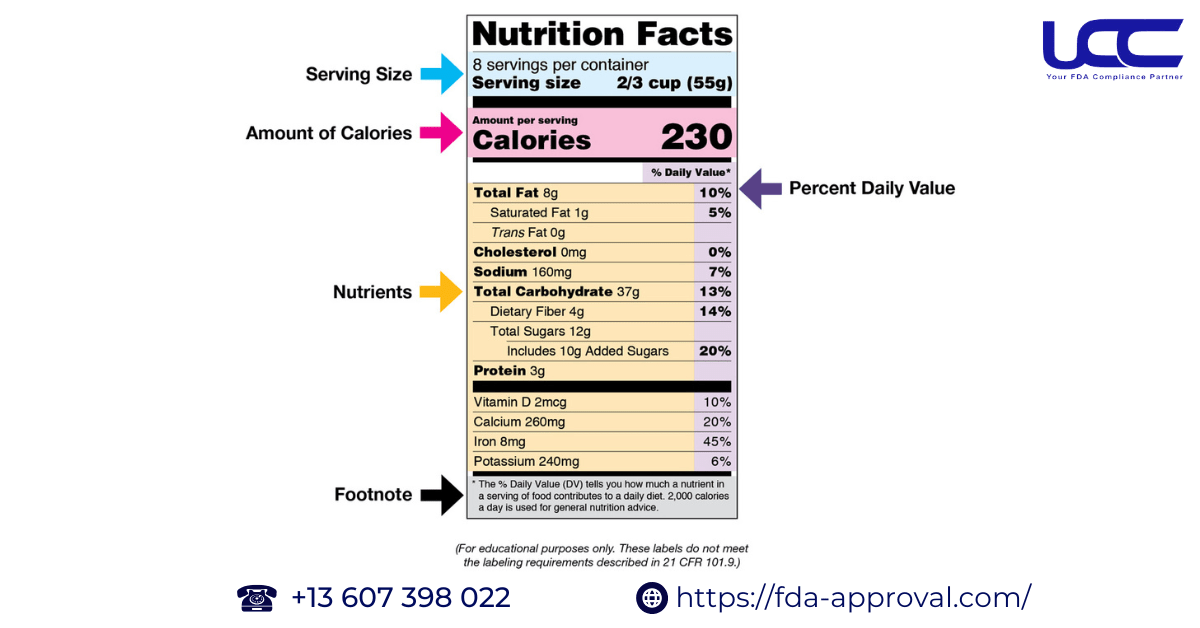
- Part 1: Serving Size
- Part 2: Calories
- Part 3: Nutrients to Limit: Total Fat, Cholesterol, Sodium, Carbohydrates, Protein
- Part 4: Nutrients to Encourage: Vitamins and Minerals
- Part 5: Note at the bottom of the nutrition facts table
- Part 6: Nutritional Information for Daily Values
2.2.2. Allergen Labeling
Each year in the U.S., thousands of cases of food allergies are reported. Allergens that are not properly communicated to consumers can have serious health consequences or even lead to death. Therefore, the FDA requires manufacturers to clearly label any ingredients that may cause allergies in order to inform consumers and help prevent unfortunate incidents.
2.2.3. Nutrition claims
Types of Health Claims Permitted on Food Labels
- Ingredients: Claims can include natural, artificial, organic, GMO, irradiated, or free from specific ingredients.
- Health: Claims can state the product is rich in beneficial substances or low in harmful components.
- Nutrition: Claims may highlight energy sources, vitamin and mineral content, or support specific dietary needs.
- Allergens/Tolerance: Claims can warn about allergens and provide tolerance information.
- Certification: Claims can confirm compliance, certification, vegan status, organic, or non-GMO attributes.
2.2.4. Food Safety Warning Labeling
The U.S. Food and Drug Administration (FDA) issues regulations regarding food safety warnings to protect consumers from potential hazards associated with certain foods. Furthermore, below are the main types of food safety warnings that must be included on food labels:
- Allergens
- Choking hazards
- Microbial risks
- Toxic substances, genetically modified organisms (GMOs), and carcinogens
2.2.5. Certification Labeling
There are many different types of product certifications, each with its own unique significance and value. In addition, below are some common system and product certifications that are often found on food labels, including certifications for:
- Origin: USDA Organic, Certified Naturally Grown, Local Food…
- Production: Fairtrade International, Rainforest Alliance Certified, Marine Stewardship Council (MSC)…
- Quality: Non-GMO Project Verified, USDA Grade, Certified Gluten-Free…
2.2.6. Small Business Nutrition Labeling Exemption
A waiver is granted for low-volume products if the applicant, according to the FDA, employs fewer than 100 full-time equivalent employees and sells less than 100,000 units of the product in the United States within a 12-month period. To qualify for this waiver, the applicant must therefore submit an annual notice to the FDA. Furthermore, it is important to note that low-volume products with nutritional claims do not qualify for this type of exemption.
2.3. Important considerations for FDA food labeling

- Product Recall
The FDA has the authority to recall food products if labeling is misleading, inaccurate, or incomplete. This may happen when companies fail to include essential information, such as allergens or nutritional content, or make false claims about their products. In such cases, Food Label Consultants can assist companies in navigating the recall process. When a recall is initiated, companies typically inform consumers, retailers, and distributors to ensure the affected product is promptly removed from the market.
Moreover, a product recall can have serious consequences for the manufacturer. Not only does it risk significant damage to the company’s reputation, but it can also lead to substantial financial losses. The costs associated with a recall may include the loss of the product itself, as well as expenses for public relations efforts, legal fees, and potential compensations for affected consumers. Furthermore, repeated recalls can result in a loss of consumer trust, which can take years to restore.
- Fines
In addition to the potential for product recalls, the FDA possesses the authority to impose fines on food manufacturers that violate labeling regulations. These fines can vary in amount, depending on the severity of the violation, and they serve as a significant deterrent for companies that fail to comply with FDA standards. Common violations, such as mislabeling ingredients, failing to disclose allergens, or not adhering to the required format for nutritional information, can lead to these penalties.
Such penalties not only represent a financial burden for manufacturers, but they also underscore the importance of compliance with food labeling laws. Therefore, we encourage manufacturers to implement rigorous quality control measures to ensure their labeling meets all FDA requirements. By doing so, they can effectively avoid both recalls and fines, thereby protecting their reputation and financial stability.
2.4. Key Changes to the Updated Food Label
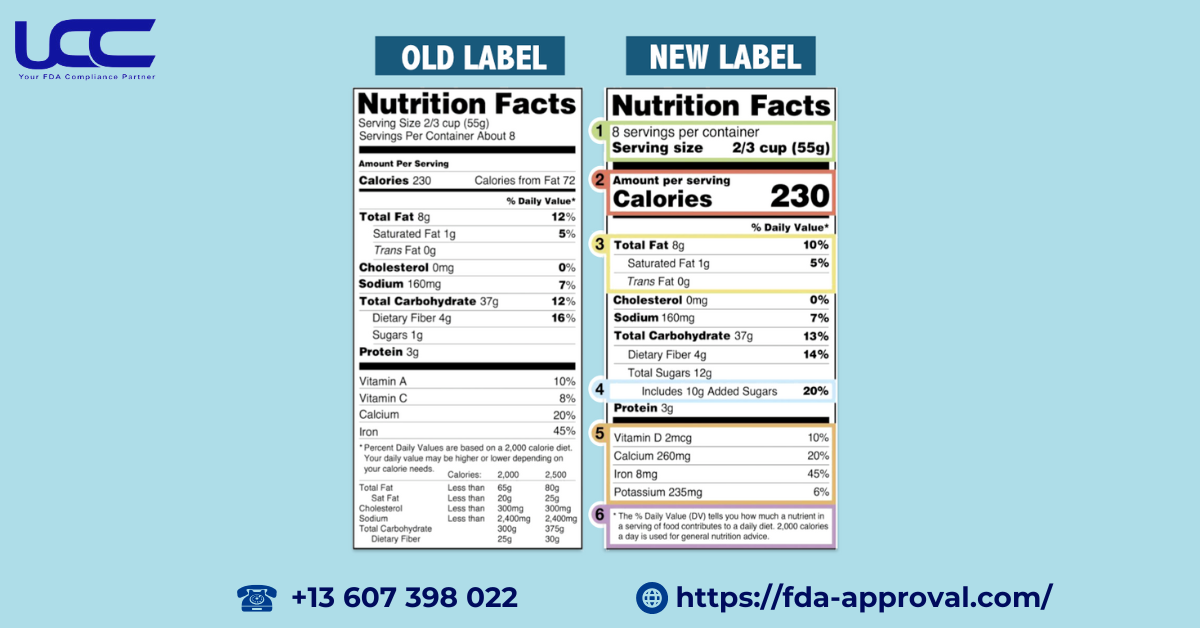
The updated FDA food nutrition labeling brings several important changes to help you make informed choices:
- Larger Font: Key details like servings per container, serving size, and calories are now more prominent, making it easier to find crucial information at a glance.
- Updated Serving Sizes: Serving sizes have been adjusted to reflect what a typical American would actually consume, ensuring that the information is relevant and realistic.
- Bold Highlights: Serving size and calorie counts are now bolded, drawing your attention to these vital pieces of information.
- Revised Nutritional Values: Daily values for sodium, dietary fiber, and vitamin D have been updated based on the latest scientific research, ensuring you have the most accurate information.
- Removed Calories from Fat: The label no longer includes a separate category for calories from fat, shifting the focus to the types of fat consumed rather than just the quantity.
- New Sugar Categories: A new category for “Added Sugars” has been introduced under Total Sugars, helping you understand the difference between naturally occurring and added sugars.
- Essential Vitamins and Minerals: Vitamin D and potassium will now be required on the label, while vitamins A and C will be listed voluntarily, allowing you to better monitor your nutrient intake.
3. Why Choose FDA Food Label Consultants Services At UCC
With over 15 years of experience, UCC is a leading provider of comprehensive FDA registration services. We not only assist businesses in completing legal procedures, but we also offer in-depth labeling consultations to ensure your products meet the stringent standards of the U.S. market. This not only builds consumer trust, but it also opens up expansive business opportunities. Additionally, we provide food labeling consultations, including FDA food nutrition labeling compliance, to ensure your products meet all necessary requirements.
Why Choose UCC group as Your Food Label Consultants?
- Extensive Expertise: Our team of experts has a thorough understanding of FDA regulations, and they continuously update their knowledge with the latest changes.
- Complete Services: From initial consultations and document preparation to ongoing support in communicating with the FDA, we accompany you throughout the entire process.
- Optimized Solutions: Additionally, we offer flexible solutions that are tailored to each product type and business scale.

For more information: Food Supplement Label: Must-Know Rules!
Invest in the future of your business with the professional FDA registration services offered by UCC. What’s more, contact us today to receive the most dedicated consultation!



