What is De Novo?
The De Novo Classification Request (De Novo Request) is a regulatory pathway for low-to-moderate-risk novel medical devices that do not have a predicate device. This pathway allows companies to bring new and innovative medical devices to market. This pathway is for devices that have a novel design or intended use and have not been classified under existing regulations.
For the De Novo Request pathway, the FDA expects the manufacturer to prove the safety and effectiveness of a medical device through the implementation of general controls alone or general and special controls. If a De Novo Request is approved by the FDA, the medical device will be classified as class I or Class II depending on the device’s risk level.
In 2021, FDA issued a final rule on the requirements for the medical device De Novo Request under the Federal Food, Drug, and Cosmetic Act (FD&C Act).
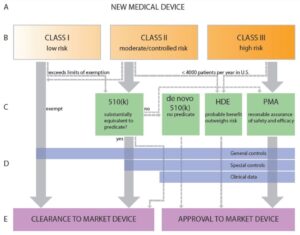
De Novo Request Submission Process
When is a De Novo Request Applicable?
A medical device may be eligible for a De Novo Request if a manufacturer determines that the device under development does not have a predicate device and that it meets the expected standards for classifying a device into Class I or Class II. This means that the manufacturer determines the device to be of low or moderate risk and believes that safety and effectiveness of the device can be determined through implementing general controls or general and special controls. FDA will primarily evaluate the risks associated with the device to assign a classification. If the FDA finds evidence for a low-to-moderate risk device to be inadequate, the device will be classified as Class III and additional testing may be required. The medical device may have to go through the more stringent PMA submission pathway to get approval. If the FDA determines the device under review to have a predicate device, the De Novo Request will be declined, and the MFG will instead submit a 510(k).
A medical device may also be eligible for De Novo Request if the medical device was originally seeking approval under a 510(k) submission, but the FDA determined the device to be non-substantially equivalent (NSE) due to lack of an identifiable appropriate predicate device.
Though not required, the FDA encourages manufacturers to submit a pre-submission for a De Novo Request as the agency can provide feedback on whether the device may be eligible for the De Novo classification process. A pre-submission can provide advantage by getting FDA’s preliminary perspective on potential regulatory controls needed for the medical device and by getting feedback on test methods required to collect clinical/nonclinical data to support De Novo submission. More details on the pre-submission can be found in the De Novo Classification Process guidance document.
De Novo Request Review Process
Acceptance Review
Once the De Novo Request is submitted to the FDA, it goes through a preliminary round of review known as the acceptance review. At this stage, the FDA reviews the submission against the acceptance checklist in the Appendix A of Acceptance Review for De Novo Classification Request guidance document to determine if the submission contains all the important information necessary to proceed to a substantive review stage. The FDA completes the review process within 15 calendar days of submission. The FDA may request additional information if it’s missing from the submission. The FDA will then pause the review and expect the requested information to be provided within 180 calendar days. If the requested information is not provided in a timely manner, the De Novo Request will be considered as withdrawn.
Substantive Review
During the substantive review, The FDA will conduct a review to determine if the device under review has a predicate device or if it falls under Class III classification. The medical device is not eligible for De Novo Request if the FDA determines that there is an existing predicate device or if the device should be classified as Class III. The FDA will also review the submitted data to evaluate if the general and/or special controls are sufficient to provide a reasonable assurance of safety and effectiveness. If the De Novo Request is accepted, the FDA will issue a written acceptance of the De Novo request which will include the classification of the device as well (Class I or Class II). More details on the substantive review can be found in the De Novo Classification Process guidance document.
Why Choose UCC for your De Novo Request Consulting Services?
At UCC, we have extensive experience navigating the De Novo Request process and can help guide your company through the submission process. Our team of experts includes former FDA reviewers who have a deep understanding of the agency’s requirements and can provide valuable insights into how to strategically position your device for success. If you are interested in learning more about UCC’s De Novo Request consulting services and how we can help you bring your medical device to market, please contact us directly to discuss your specific needs.
To learn more about UCC’s De Novo request process consulting services, contact us to discuss your specific needs.



