For medical device traders looking to enter the U.S. market, the 510(k) process is a critical step for FDA review and approval. Therefore, what exactly is 510(k)? Moreover, is it mandatory for everyone to register a 510(k)? In this article, let’s explore Pre-Market Notification in detail with UCC!

1. Overview of FDA’s Pre-Market Notification 510(k)
The FDA’s Premarket Notification 510(k), also known simply as 510(k), is a submission process to ensure medical devices meet safety and effectiveness standards before they enter the U.S. market. Besides, the term “Premarket Notification” (PMN) directly references section 510(k) of the FDA standards. Specifically, the 510(k) is a pre-market submission that shows the device is substantially equivalent to an existing, legally marketed device in terms of safety and effectiveness (Section 513(i)(1)(A) of the FD&C Act).

2. Who Must Submit a 510(k) Pre-Market Notification?
2.1. Entities Required to Submit a 510(k)

Several types of entities are required to submit a 510(k) notification to the FDA before marketing a device in the U.S.:
- Domestic Manufacturers
Domestic manufacturers that create and market a finished device in the U.S. must submit a 510(k) if the device specifications are unique to their design and intended for the U.S. market. Accessories that go to end-users are also considered finished devices and thus require a 510(k). However, component manufacturers do not need to submit a 510(k) unless their components are marketed directly as replacements for end-users. - Specification Developers
Specification developers who develop the device specifications for contract-manufactured devices must submit a 510(k) instead of the contract manufacturer. - Repackagers or Relabelers
Those who repackage or relabel a device may need to submit a 510(k) pre-market notification if their changes affect the device’s significant properties. - Foreign Manufacturers and Exporters Looking to Enter the U.S. Market
Foreign manufacturers and their U.S. representatives must submit a 510(k) for devices they introduce to the U.S. market.
2.2. Exemptions from the 510(k) Submission Requirement
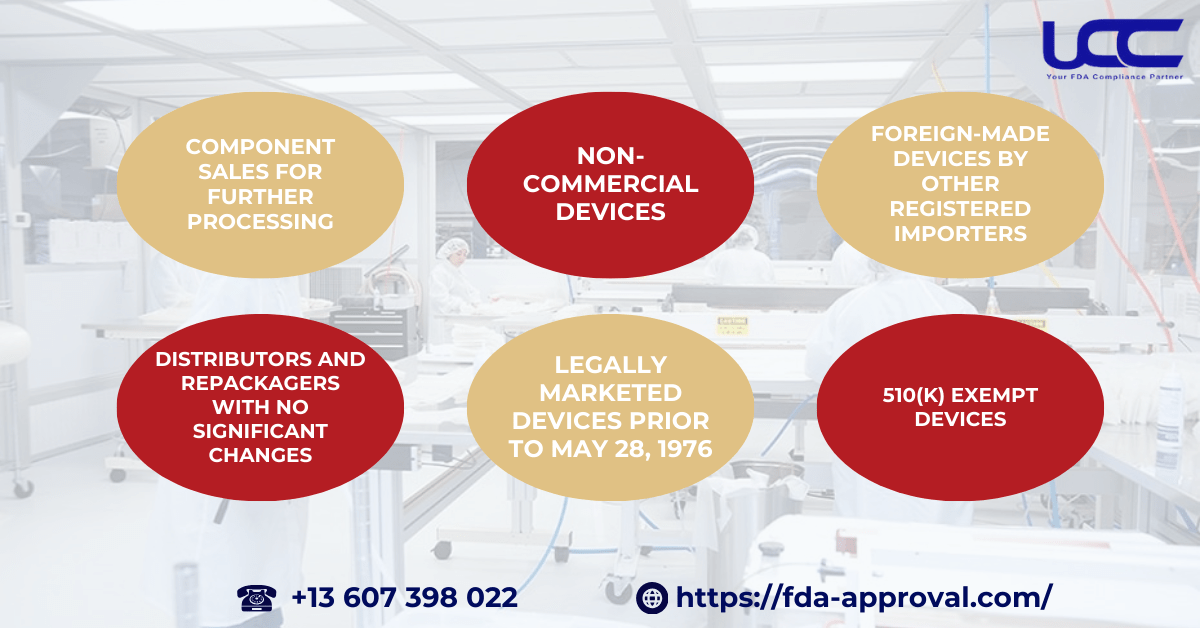
Certain situations exempt an entity from needing a 510(k) pre-market notification:
- Component Sales for Further Processing
If a company sells unfinished devices or components to others for further processing or as part of a larger assembly, they do not need to submit a 510(k). However, if components are sold directly to end-users as replacements, a 510(k) pre-market notification is required. - Non-Commercial Devices
If your device is not intended for marketing or commercial distribution, then you do not need a 510(k). This specifically includes devices that are used solely for development, testing, and clinical evaluations. Therefore, if your purpose aligns with these categories, the 510(k) requirement does not apply. - Distributors and Repackagers with No Significant Changes
Distributors of domestically manufactured devices who do not alter significant labeling elements generally do not need to submit a 510(k). - Legally Marketed Devices Prior to May 28, 1976
Devices lawfully distributed before May 28, 1976, without significant modifications, are exempt from the 510(k) requirement. - Foreign-Made Devices by Other Registered Importers
Importers of foreign-made devices do not need to submit a 510(k) if the foreign manufacturer already has an approved 510(k). - 510(k) Exempt Devices
Certain Class I and Class II devices as specified in 21 CFR 862-892 may enter the U.S. market without a 510(k) pre-market notification.
3. Pre-market Notification 510(k) Submission Process
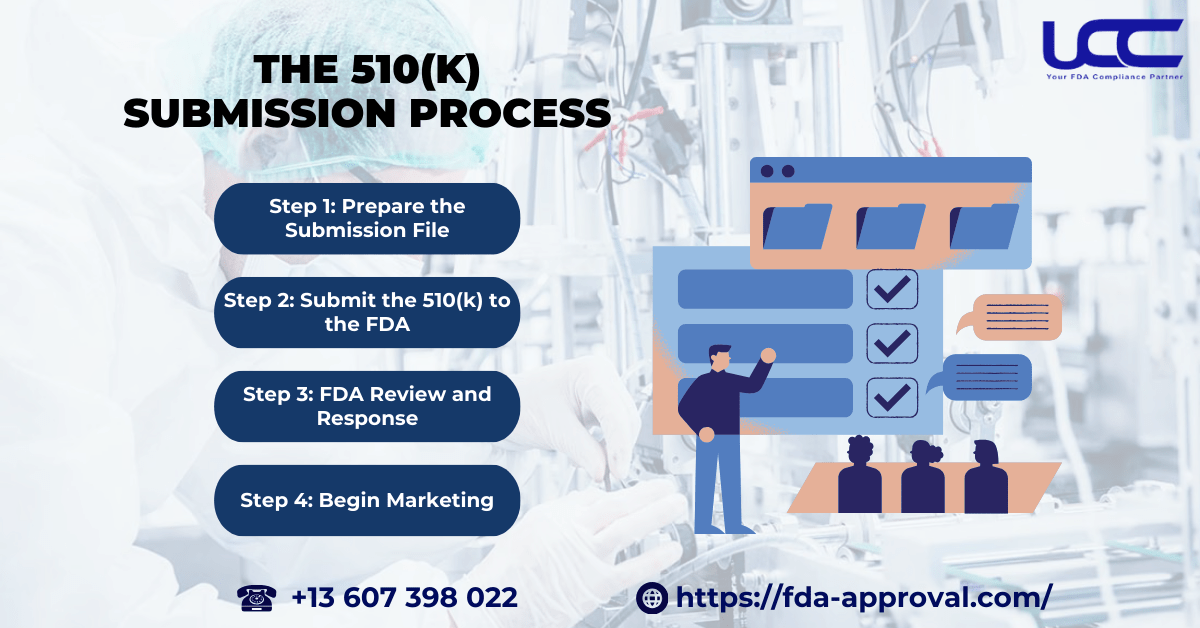
Submitting a 510(k) can be complex, but it is crucial for legally marketing a medical device in the U.S. The 510(k) registration process will unfold in the following manner:
Step 1: Prepare the Submission File
The Premarket Notification submission file should include detailed information about the medical device, such as:
- Device Description
This section should describe the device’s structure, function, specifications, and usage. - Performance Data
Include data supporting the device’s safety and effectiveness. - Comparison Information
Compare your device with a legally marketed device already cleared by the FDA. - Manufacturing Plan
Provide a detailed description of the manufacturing process. - Labeling Information
Include labeling and usage instructions for the device. - Declaration of Truth and Accuracy
This should be included in the 510(k) cover letter or as a separate item in the table of contents. - 510(k) Summary or Statement
Include a summary or statement about the device’s substantial equivalence.
Step 2: Submit the 510(k) to the FDA
As of October 1, 2023, all 510(k) pre-market notification submissions must be electronic via eSTAR, which is an interactive PDF designed for creating comprehensive pre-market submissions. Furthermore, this transition to electronic submissions aims to streamline the process and improve efficiency in the review system.
Step 3: FDA Review and Response
- Day 1: The FDA receives the 510(k) submission.
- By Day 7: The FDA sends a confirmation or cost issue notice.
- By Day 15: The FDA conducts an acceptance review; subsequently, it notifies the applicant of any hold for content review.
- By Day 60: The FDA performs a substantive review, informing applicants of the need for further information or approval.
- By Day 90: The FDA issues the final decision on the 510(k).
- By Day 100: If no decision is issued by Day 100, the FDA will notify the applicant of any remaining issues for resolution.
Step 4: Begin Marketing
Once the FDA approves the 510(k), you can legally market and sell your medical device in the U.S.
4. Important Considerations for 510(k) Pre-Market Notification Submissions
-
Submission Format
Starting October 1, 2023, all 510(k) forms must be submitted electronically through eSTAR. It’s also necessary to submit the 510(k) at least 90 days before product launch.
-
510(k) Substantial Equivalence Requirements
The 510(k) process requires demonstrating “substantial equivalence” with a legally marketed device. A device is substantially equivalent if it has the same intended use and technological characteristics or, with different technology, poses no new safety or effectiveness questions and meets safety and performance standards.
Using the 510(k) Pre-Market Notification process allows medical device manufacturers to confidently and legally bring their innovations to the U.S. market. By following these guidelines, companies can streamline the path to FDA compliance, ensuring timely and successful market entry.
We hope this article helps you better understand 510(k) and the applicable entities. Additionally, UCC currently offers consultation services for 510(k) submissions. If you have any questions or need further assistance, please do not hesitate to contact us via our hotline at +1 3607 398 022 to receive the most attentive support!



