The registration of cosmetic facilities was previously a voluntary process for businesses. However, since 2022, cosmetics businesses must also complete another registration: the cosmetic facility registration. In this article, UCC will provide important information regarding cosmetic facility registration.

1. Cosmetic Facility Registration Overview
The passage of the 2022 law marks a significant milestone in cosmetics regulation in the United States. It represents the most substantial expansion of the FDA’s authority over cosmetics since the Federal Food, Drug, and Cosmetic Act (FD&C Act) was enacted in 1938.
Under the Modernization of Cosmetic Regulation Act (MoCRA) approved in 2022, the FDA mandates that most cosmetic facilities, including contract manufacturers producing cosmetics for the U.S. market, register with them. Besides, registered facilities must promptly update their registration details and renew their registration every two years. Additionally, all foreign facilities must designate a U.S. agent to work with the FDA.
2. Who Needs to Register for Cosmetic Facility Registration?
Anyone who owns or operates a facility involved in manufacturing or processing cosmetic products for distribution in the United States must register each facility, except in certain specified cases. This requirement applies to companies both domestically and internationally.

3. Required Information
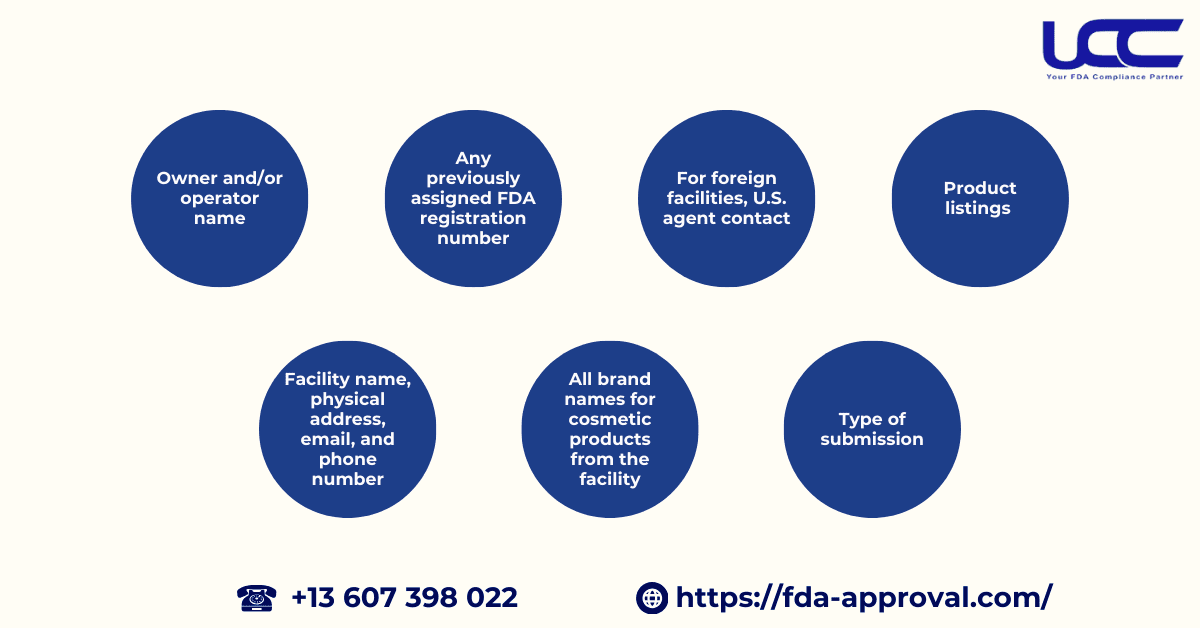
According to sections 607(a) and 607(b)(2) of the FD&C Act, the following information must be submitted in the facility registration application:
- The name of the owner and/or operator of the facility;
- The name of the facility, along with its physical address, email address, and phone number;
- For any foreign facility, contact information for the U.S. representative (name and phone number), and if available, electronic contact information (email);
- Any previously assigned registration number for the facility from the FDA;
- List all brand names under which your facility sells the cosmetic products it manufactures or processes;
- The product listing or categories for each cosmetic product manufactured or processed at the facility, along with the responsible person;
- The type of submission (initial, amended, biennial renewal, or abbreviated renewal).
Additionally, the registration process involves two key steps. First, it is essential for the facility to register and obtain its FDA Establishment Identifier (FEI number), which serves as a unique identification for the establishment. Second, the facility must submit registration information.
4. How to Find a Reliable U.S. Representative?
The cosmetic facility registration process is crucial for ensuring compliance and meeting regulatory requirements. This step also helps convey professionalism and safeguard consumer health when using your products. To begin, you need a reputable U.S. representative.
When selecting a U.S. representative, consider various factors, such as cost, reputation, and response time. UCC confidently offers over 15 years of experience and thousands of partners worldwide. Our experienced team can handle any arising situation effectively. Moreover, upon successful registration with the FDA, the U.S. representative – UCC – will be the direct entity responsible for issuing the FDA certificate to the business. As the direct authority, UCC ensures that the FDA registration certificate is delivered to you in a timely manner. Our approach centers on understanding customer needs and delivering personalized solutions. Contact us today to make your entry into the U.S. market easier!

We hope this article provides a comprehensive overview of cosmetic facility registration. Stay tuned for more articles from UCC. For any inquiries or further clarification, please contact UCC!



