When seeking to do business in the world’s leading market—the United States—you must comply with certain FDA labeling requirements. Currently, Food supplements are quite familiar to consumers. So, what regulations apply to Food Supplement Label, and what should you keep in mind? In this article, UCC will help you better understand these requirements to ensure your product complies with FDA regulations!

1. What Is A Food Supplement?
A Food supplement refers to a product (excluding tobacco) that contains nutrients derived from food, intended to supplement the diet. Vitamin and mineral supplements are the most common types of dietary supplements, used to treat deficiencies or prevent health issues. Other Food supplements include protein supplements, omega-3 fatty acid supplements, and herbal supplements, which you may encounter in your daily life.
In everyday life, Food supplements come in various forms, such as tablets, gummies, soft gels, and liquids. It is important to note that dietary supplements are neither food nor medicine; thus, individuals should use them only as part of a healthy lifestyle.

So, what constitutes the labeling of Food supplements?
Firstly, it is essential to assert that labeling is a broad term. It applies not only to the physical labels affixed to the containers but also to marketing materials accompanying the Food supplement or claims made on the product’s sales website. In the following sections, we will delve deeper into the general requirements for Food Supplement Label.
2. General Requirements For Food Supplement Label

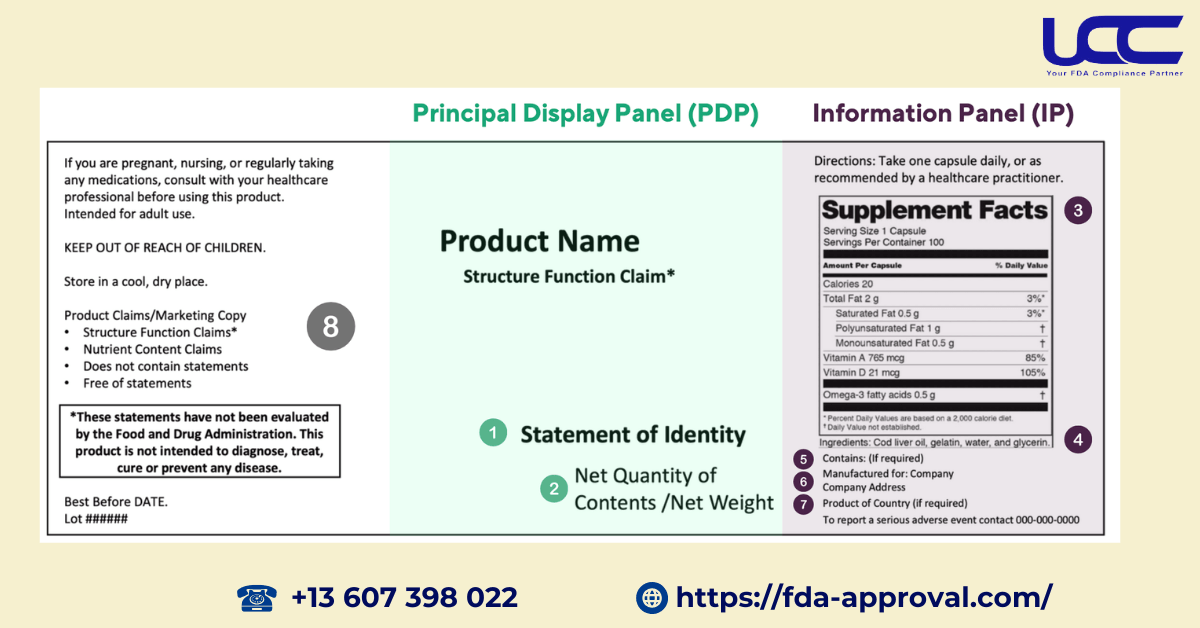
Every dietary supplement product must meet specific basic labeling requirements to be sold in the United States, including:
- Identity Statement: The label must state “Food supplement” or “supplement” before the specific ingredient type, such as “Food supplement of fiber.”
- Net Weight: The net weight of the contents must be clearly indicated.
- Ingredient List: A comprehensive list of all ingredients in the product, also including all FDA Color Additive Requirements, must be provided.
- Manufacturer Information: The label must include the manufacturer’s, packager’s, or distributor’s name and address, specifying the company name and street address (unless a local address is provided).
- Consumer Contact Information: A phone number or mailing address in the United States where consumers can report serious adverse events must be provided.
- Supplement Facts: The label must clearly indicate “Serving Size” and “Servings Per Container” if this information is not included in the net weight section.
UCC emphasizes that “Serving Size” must reflect the maximum recommended amount “per serving.” For instance, if the instructions state two capsules before meals, the serving size will be two capsules.
3. Specific Labeling Requirements For Food Supplement Ingredients
- Ingredient List
- Allergen Statements
Common allergens typically include ingredients such as milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, and soy. Clear allergen declarations help consumers who are allergic or sensitive to certain foods avoid products that may trigger adverse reactions, thus minimizing unwanted risks.
- Nutritional Information
Accurate nutritional information on Food Supplement Labels is essential. FDA regulations require serving size, servings per container, total calories, macronutrients (carbohydrates, protein, fats), and essential vitamins and minerals to help consumers understand the product’s nutritional value.
- FDA New Dietary Ingredient (NDI)
The FD&C Act defines “New Dietary Ingredients” (NDI) as ingredients that manufacturers have not marketed in the United States in dietary supplements prior to October 15, 1994. Any manufacturer or distributor intending to market dietary supplements containing “New Dietary Ingredients” in the U.S. must submit a notification to the FDA regarding these ingredients.
4. Claims on Dietary Supplement Labels
The FDA clarifies which claims on Food Supplement Label may be considered inappropriate drug claims under Federal Code 21, 101.93(g). A claim becomes inappropriate if it explicitly or implicitly suggests that the product directly affects a specific disease, its symptoms, or an abnormal condition, particularly if the condition is uncommon or could lead to significant or permanent harm.
Some claims regulated by the FDA include:
- Nutrient Content Claims: These are characterized by the level of nutrients.
- Health Claims: These describe a reduced risk of disease associated with the consumption of a specific nutrient. For example, the FDA allows the claim that consuming oats may lower the risk of heart disease.
- Structure/Function Claims: These describe the role or mechanism of a nutrient’s effect on the structure or function of the body.
- Drug Claims: These imply that a Food supplement may diagnose, alleviate, treat, cure, or prevent specific diseases or groups of diseases. The FDA prohibits Food supplement labels from including drug claims. Labels that imply treatment effects, such as “reducing severe chest pain,” are prohibited. If a dietary supplement includes drug claims, the FDA may classify it as a drug, even without active pharmaceutical ingredients.

5. FDA Enforcement Actions
In February 2021, the FDA announced that it sent warning letters to ten Food supplement manufacturers making claims that their products could treat or cure depression and other mental health disorders. This exemplifies the FDA’s enforcement actions.
The FDA can issue warning letters or place a company on Import Alert if it finds a product to be unapproved. This may occur due to drug claims on a Food supplement’s labeling. If a company is on the Import Alert list, customs may detain its products without physical examination. The importer must request FDA review and demonstrate compliance to allow shipments at U.S. entry ports.
Thus, the seriousness of failing to comply with Food Supplement Label regulations is evident. You should be extremely cautious and attentive when designing the labels for these products.
In conclusion, navigating the landscape of Food Supplement Label in the U.S. is essential for ensuring compliance with FDA regulations. By understanding the specific requirements and guidelines, you can effectively position your product in the market while safeguarding consumer health. UCC is here to support you, providing guidance and resources to successfully launch your dietary supplement.



