The terms “Supplement Facts” and “Nutrition Facts” often lead to confusion when it comes to labeling products in compliance with FDA regulations. In this article, UCC aims to clarify what Supplement Facts are and outline the specific FDA requirements for these labels.
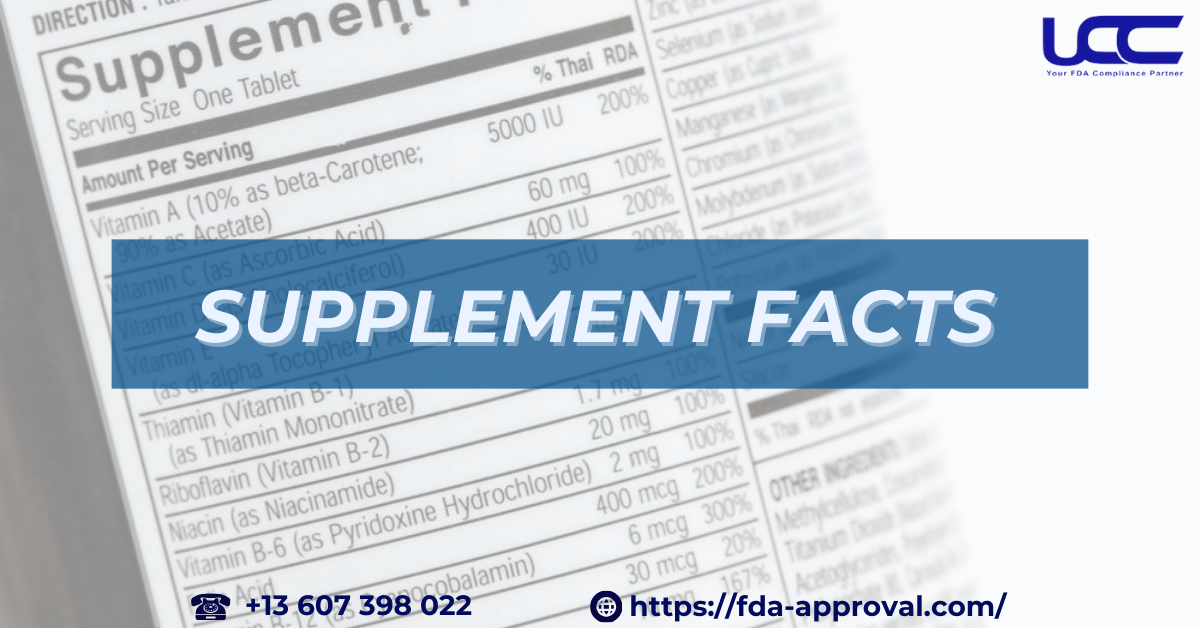
1. Overview of the Supplement Facts
Supplement Facts is a crucial section on the label of dietary supplements. It provides detailed information regarding the active ingredients and their quantities per serving. The primary aim of this section is to enable consumers to make informed and educated decisions regarding the supplements they choose to consume. The ingredients listed typically include vitamins, minerals, amino acids, fatty acids, and other supplemental compounds, along with their precise quantities.

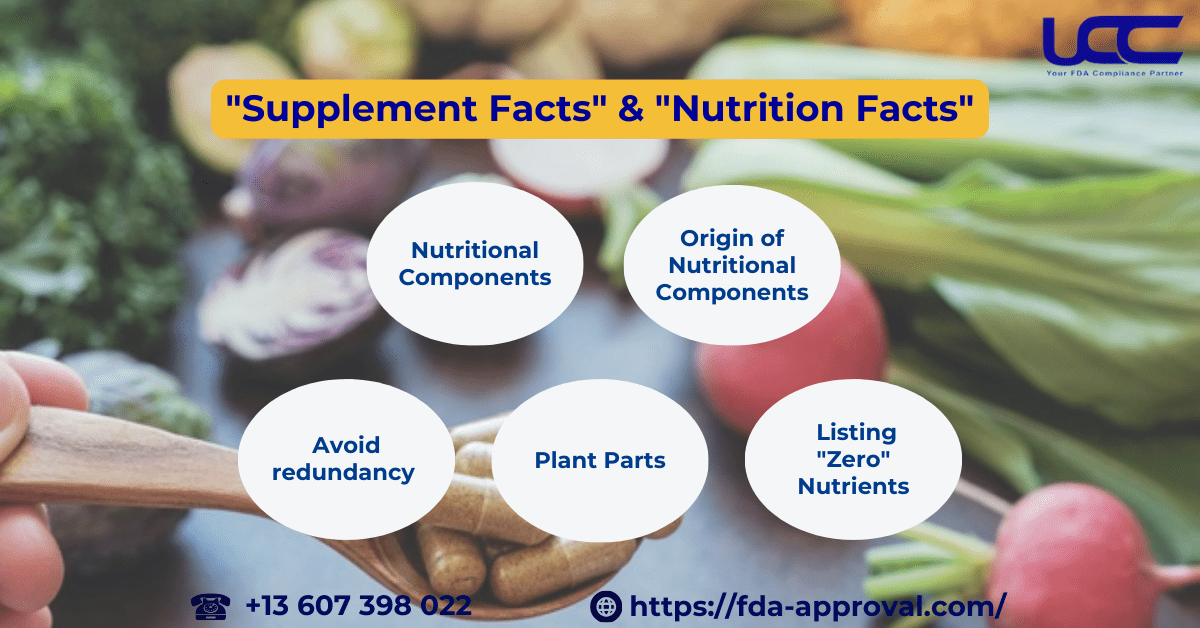
2. Distinguishing Between “Supplement Facts” and “Nutrition Facts”
2.1. Nutritional Components
In the “Supplement Facts” section of dietary supplements, you must list the nutritional components without including the Recommended Daily Intake (RDI) or Daily Value (DV). Furthermore, you cannot include these components in the “Nutrition Facts” section of food product labels.
2.2. Origin of Nutritional Components
It is permissible to list the source of nutritional components in the “Supplement Facts” section. However, the source of ingredients should not appear in the “Nutrition Facts” section of food products. For instance, the origin of a supplement, such as vitamins or minerals, should only be noted within the Supplement Facts section, not within the “Nutrition Facts” of a food product.
2.3. Avoid Redundancy in the Ingredient Statement
If you have already listed an ingredient in the “Supplement Facts” section, you do not need to repeat its source in the ingredient statement. This helps optimize the use of space on the label and prevents unnecessary duplication of information.
2.4. Plant Parts
If the nutritional component originates from a plant, you must state the specific part of the plant from which it is derived in the “Supplement Facts” section (e.g., roots, leaves, seeds). You cannot include this information in the “Nutrition Facts” section, ensuring that consumers receive accurate and detailed information about the source of the ingredient.
2.5. Listing “Zero” Nutrients
It is prohibited to list nutrients as “zero” in the Supplement Facts section of dietary supplements. In contrast, “zero” amounts for nutrients must be indicated in the “Nutrition Facts” section of food products.
Further details are available in specific FDA regulations, including 21 CFR 101.36(b)(3) and (b)(2)(i), 21 CFR 101.4(h), 21 CFR 101.36(d) and (d)(1), and 21 CFR 101.9.
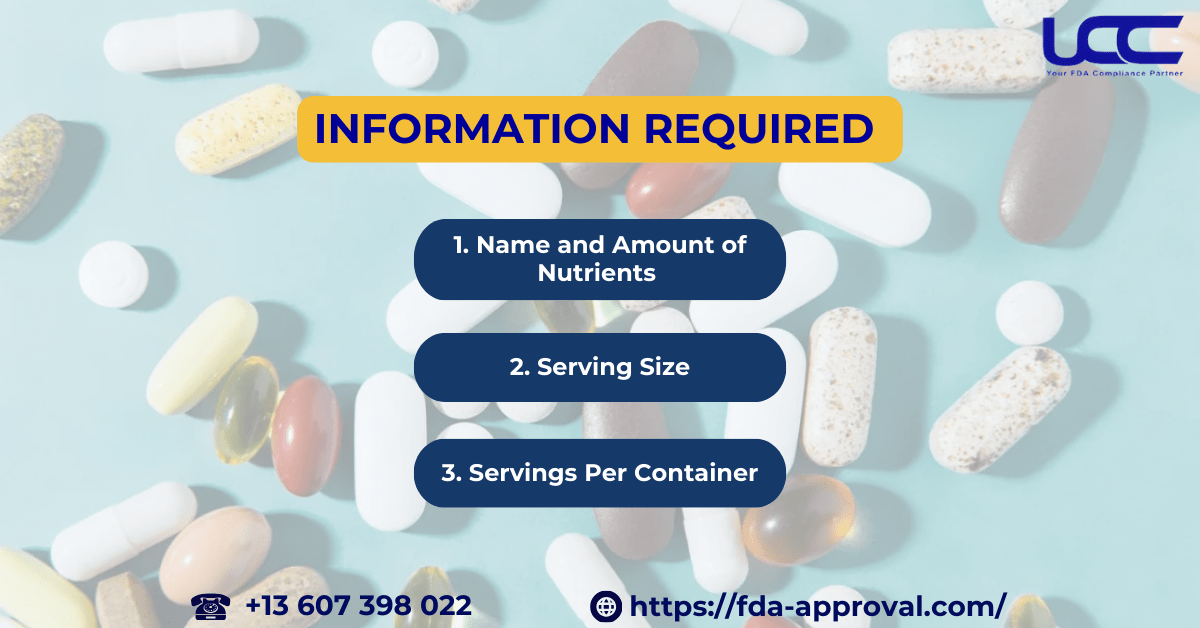
3. Information Required in the FDA Supplement Facts
3.1. Name and Amount of Nutrients
The Supplement Facts panel must include the following nutrients when they are present in substantial amounts in dietary supplements:
- Total fat
- Saturated fat
- Trans fat
- Cholesterol
- Sodium
- Total carbohydrates
- Dietary fiber
- Total sugars
- Added sugars
- Protein
- Vitamins and minerals: You must list vitamin D, calcium, iron, and potassium. Additionally, you may list other vitamins and minerals voluntarily; however, you must include them if you add them to the supplement or make health claims about their benefits.
This ensures transparency and allows consumers to make well-informed choices, as required by the FDA under 21 CFR 101.9(c).
3.2. Serving Size
The serving size indicates the amount of product you should consume in one sitting. For example, if the recommended serving is one capsule, taken three times daily, the serving size listed on the panel would be one capsule.
3.3. Servings Per Container
While you are not required to list the number of servings per container when this information matches the quantity statement, many choose to include it. For instance, if the quantity statement indicates 100 tablets and the serving size is one tablet, you would list “Servings Per Container” as 100 tablets, even though repeating this information is not necessary.
4. Key Considerations for Supplement Facts
First, if your product makes any health claims related to specific ingredients (such as saturated fats, sugars, fiber, or carbohydrates), you must list those nutrients in the Nutrition Facts panel. It is critical to disclose these nutrients accurately.
Additionally, you cannot list amino acids under the protein section in the Nutrition Facts panel. Although amino acids function similarly to proteins, you should not treat them as equivalent on the label.
By reviewing this article, we hope that you now have a clear understanding of the distinction between “Supplement Facts” and “Nutrition Facts,” as well as the necessary information that must be included in the section. Should you have any further inquiries or require assistance with FDA-compliant labeling, we kindly invite you to contact UCC at your earliest convenience.



