The FDA mandates that all packaged foods and dietary supplements must feature a nutrition label; however, there are certain circumstances under which specific exemptions may be applicable. In this article, UCC will clarify the exemptions, particularly the small business nutrition labeling exemption, and guide you on how to file an exemption request.

1. Who Qualifies for Small Business Nutrition Labeling Exemption?
According to FDA regulations, all packaged foods and dietary supplements must carry a nutrition label unless they meet specific exemption criteria. These criteria are detailed in Title 21 of the Code of Federal Regulations (21 CFR), with separate provisions for foods and dietary supplements.

1.1. FDA Exemption for Small Business Nutrition Labeling
Small businesses may qualify for a nutrition labeling exemption if they meet any of the following conditions, as outlined in 21 CFR 101.9(j)(1) and 21 CFR 101.36(h)(1):
- The business has annual gross sales not exceeding $500,000.
- Alternatively, its food or dietary supplement sales directly to consumers do not exceed $50,000 annually.
In these cases, businesses do not need to notify the FDA.
1.2. FDA Exemption for Low-Volume Products
For products with low sales volume, businesses may qualify for an exemption if they meet the following requirements under 21 CFR 101.9(j)(18) and 21 CFR 101.36(h)(2):
- The business employs fewer than 100 full-time employees on average.
- It sells fewer than 100,000 units of the product in the U.S. within a 12-month period.
For this exemption, businesses must submit an annual notice to the FDA to maintain their exempt status.
1.3. FDA Exemption for Very Small Businesses
Businesses that are not importers and employ fewer than 10 full-time employees can qualify for an exemption without submitting a notice if they sell fewer than 10,000 units of a product annually.
2. How to File for a Small Business Nutrition Labeling Exemption
2.1. Information Required for Filing FDA Exemption
To file for a small business nutrition labeling exemption, businesses must provide the following details:
- Company Information: Name, address, phone number, fax, and email.
- Type of Business: Specify whether the business is a manufacturer, packer, retailer, distributor, or importer.
- Exemption Period: Clearly state the 12-month exemption period (e.g., May 8, 2023 – May 7, 2024).
- Employee and Product Volume: Provide the average number of full-time employees and the number of products sold or estimated to be sold.
- Product Details: Include the product name, quantity sold, and information about the manufacturer, distributor, or importer (if different).
- Contact Person: Name and phone number of the person responsible.
- Certification: A signed declaration confirming the accuracy of the submitted information.
Note: The responsible person must notify the FDA if the employee count or sales volume exceeds the exemption limits during the exemption period.
2.2. Methods of Submission
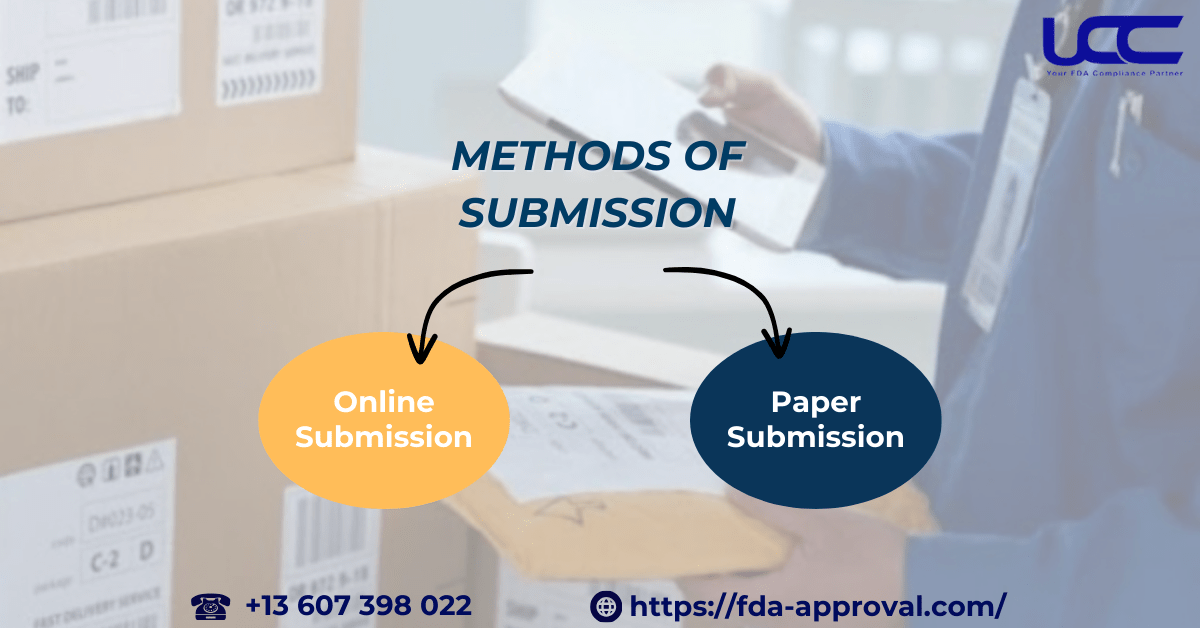
Businesses can submit their exemption notices to the FDA through two methods:
- Online Submission
Using the FDA’s web-based submission system offers a convenient and fast way to file notices. This method saves time and ensures quick delivery.
- Paper Submission
For businesses unfamiliar with online systems or facing internet challenges, the FDA still accepts paper submissions via mail or fax. Businesses can use the FDA’s Small Business Exemption Notification Form for this process.
3. Important Notes on Small Business Nutrition Labeling Exemption Filing
3.1. FDA Limitations on Labeling Claims
Businesses lose their nutrition labeling exemption if their product labels, packaging, or advertising include:
- Nutrient content claims (e.g., “sugar-free,” “low-fat”).
- Health claims (e.g., “heart-healthy”).
- Any additional nutritional details.
In such cases, businesses must comply with all FDA nutrition labeling requirements, regardless of size or sales volume.
Additionally, the exemptions apply only to nutrition labels and do not affect other mandatory label information, such as:
- Identity Statement: A clear product name.
- Net Weight: The total weight or volume of the food product excluding packaging.
- Ingredient List: All ingredients listed in descending order by weight.
- Manufacturer Information: The name and contact details of the producer, packager, or distributor.
3.2. FDA Annual Notification Obligation
Businesses must submit an updated exemption notice to the FDA each year to maintain their exempt status. If the FDA does not receive this notice, the exemption automatically expires.
Key Points to Remember:
- The FDA does not send reminders for filing deadlines.
- The FDA does not confirm receipt of your notice.
To protect your business, keep a copy of your submitted notice for your records.
For more information: How to Register FDA Food Facility: A Step-by-Step Guide
We trust this guide has provided you with a comprehensive understanding of the small business nutrition labeling exemption and other related FDA exemptions. Should you have any further inquiries or require professional assistance with FDA compliance, please do not hesitate to reach out to UCC.



