The Food Safety Modernization Act (FSMA), enacted by the FDA, establishes rigorous requirements to proactively address food safety risks. Within this framework, the “Final Rule for Preventive Controls for Human Food” mandates that food facilities implement preventive measures and adhere to Current Good Manufacturing Practices (CGMP), with certain exemptions. In this article, UCC presents essential information regarding preventive controls for human food.

1. Overview of Preventive Controls for Human Food
1.1. Definition
The rule governing preventive controls for human food became effective in September 2015. Specifically, it requires food facilities to establish a food safety plan, including hazard analysis and risk-based preventive controls, to mitigate or prevent identified hazards. This rule covers several key areas, including personnel, equipment, facilities, sanitation, and comprehensive food safety planning.

Furthermore, food facilities must adhere to Current Good Manufacturing Practices (CGMP) to ensure the hygiene and quality of their products. Notably, all facilities producing food for U.S. consumption must comply with CGMP, even if they are not registered with the FDA, with few exceptions.
1.2. Purpose
The “Current Good Manufacturing Practices, Hazard Analysis, and Risk-Based Preventive Controls for Human Food” rule safeguards the production, processing, packaging, and storage of food products. This rule applies to food products intended for human consumption in the United States.
The rule on preventive controls for human food ensures that facilities take appropriate and proactive measures to minimize the risk of food contamination. Both domestic and foreign food facilities that provide products to the U.S. market must register with the FDA under Section 415 of the Food, Drug, and Cosmetic Act (FDCA) and comply with FSMA’s preventive control mandates. FSMA focuses on preventive measures to avoid food safety incidents rather than reacting after they occur.
2. Application Scope of Preventive Controls for Human Food
The requirements generally apply to entities involved in the manufacturing, processing, packaging, or storage of food for human consumption in the United States. This encompasses both domestic and foreign facilities that are mandated to register with the FDA under Section 415 of the Federal Food, Drug, and Cosmetic Act (FD&C).
The regulations exempt certain entities, such as “farms,” retail food establishments, and restaurants, from preventive control requirements since they are not required to register. Additionally, some exemptions or modified requirements may apply under specific conditions.

3. Mandatory Provisions of Preventive Controls for Human Food
The revisions have made certain provisions, such as education and training, mandatory, which were previously non-mandatory. The specific requirements are as follows:
- Management must ensure that all personnel involved in the manufacturing, processing, packaging, or storage of food products are appropriately qualified to perform their assigned duties.
- Such personnel must possess a combination of the necessary educational background and/or relevant training and experience essential for the production, processing, packaging, or storage of food products in a manner that maintains cleanliness and safety. Trainers must educate individuals on food hygiene and safety principles, including critical aspects of employee health, hygiene, food handling, and their specific responsibilities.
Additionally, the FDA’s longstanding position is that CGMP (Current Good Manufacturing Practice) addresses the issue of cross-contact with allergens as specified in the regulations. Consequently, businesses must implement measures and protocols to control cross-contact with allergens.
The CGMP now includes provisions that address the storage and distribution of food products intended for human consumption, which are later used as by-products in animal feed.
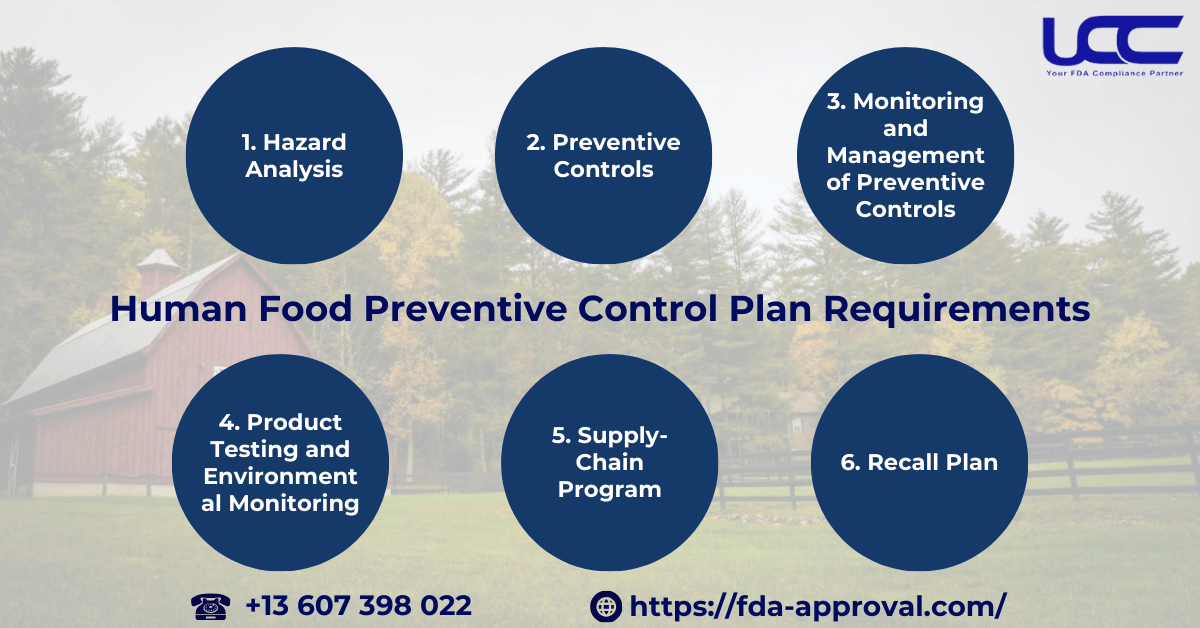
4. Preventive Controls for Human Food Plan Requirements
As outlined previously, every domestic or foreign food facility must have and implement a written food safety plan. This document identifies food safety hazards requiring preventive controls and prescribes preventive measures to significantly reduce or prevent these hazards. According to FDA regulations, the plan must include the following components:
4.1. Hazard Analysis
The initial step involves identifying hazards, which requires consideration of known or reasonably foreseeable biological, chemical, and physical hazards. These hazards may be present naturally, introduced unintentionally, or intentionally for economic reasons (if they impact food safety).
If the hazard analysis identifies any hazard that necessitates preventive controls, the facility must have and implement written preventive controls to address the identified hazards.
4.2. Preventive Controls
Facilities can tailor preventive measures to address the specific hazards in their products. They must implement written preventive controls to minimize or prevent any hazard requiring control and ensure the food remains unadulterated. The rule specifies several categories of preventive controls:
- Process Controls: Procedures to ensure control parameters are consistently met. Examples of process controls include cooking, cooling, and acidifying food, with critical limits specified in line with the role of each control in the facility’s food safety system.
- Allergen Controls: Facilities must write procedures to manage allergen cross-contact and ensure accurate allergen declarations on labels for packaged food products.
- Sanitation Controls: Procedures and practices to maintain sanitary conditions, minimizing or preventing hazards such as pathogens within the environment, hazards from food processing personnel, and allergen-related hazards.
- Other Controls: Facilities may need to implement additional controls to effectively minimize or prevent any hazard requiring preventive control.
4.3. Monitoring and Management of Preventive Controls
Once a facility identifies a preventive control for a hazard, it must effectively implement these control measures.
- Monitoring: Procedures to verify that preventive controls are consistently applied. Monitoring aligns with each preventive control. For example, monitoring thermal processes to inactivate pathogens involves recording temperature values. Facilities must maintain monitoring records.
- Correction: Steps to address minor and isolated issues promptly within the food production process.
- Corrective Action: First, steps should be taken to identify and correct issues, followed by reducing the likelihood of recurrence. Next, it is important to assess the safety of the affected food. If the safety of the food cannot be assured, it must be prevented from entering commerce. Additionally, it is necessary to document corrective actions.
- Verification: Activities to confirm that preventive controls are consistently implemented and effective in mitigating hazards. Examples include scientific validation of process preventive controls, calibration of monitoring instruments, and process verification activities like thermometer checks. Facilities must actively review records as part of verification activities to confirm effective monitoring and corrective actions (if required). Facilities must maintain verification records.
4.4. Product Testing and Environmental Monitoring
These are additional verification activities based on the food, facility, and preventive control’s role in the safety system. The facility must conduct environmental monitoring if it identifies contamination of ready-to-eat food by an environmental pathogen.
4.5. Supply-Chain Program
Manufacturers must establish a risk-based supply-chain program if hazard analysis identifies a hazard requiring control. A facility is exempt from this requirement if it controls the hazard within its own operations. Alternatively, if another entity manages the hazard, the facility must still comply with the applicable requirements.
Manufacturers must ensure they source raw materials and components from approved suppliers. In some cases, they may use unapproved suppliers, provided they verify the materials before acceptance. Additionally, a broker or distributor may conduct supplier verification, but the receiving facility must review the documentation to confirm hazard control.
4.6. Recall Plan
If hazard analysis identifies a hazard requiring preventive control, the facility must, therefore, create a written recall plan. The plan must, in turn, include procedures for notifying recipients, informing the public, conducting effectiveness checks, and managing recalled products.
For more information: Food Safety Modernization Act: Key Insights and Overview
UCC trusts that this article has provided comprehensive insights into preventive controls for human food. Should you have any questions or require further information, please contact UCC at +13607398022. We look forward to the opportunity of working together to unlock the U.S. market!



