Prior Notice is a term that is well-known among importers. In this article, UCC aims to provide a deeper understanding of Prior Notice meaning and its related information.
1. The meaning of Prior Notice
To implement the regulations of the Bioterrorism Act, the U.S. Food and Drug Administration (FDA) has issued a rule requiring prior notice for all imported food shipments into the United States. Since May 6, 2009, the FDA has required U.S. food importers to submit a Prior Notice for imported food and animal products, unless specific items are exempt from these regulations.
It can be understood as the process of registering advance information about imported food, beverages, and Food supplements. This provides the FDA with important information about food shipments arriving in the U.S., helping the agency ensure food safety for both humans and animals.
2. Essential Information Required
Certain information must be submitted to the FDA prior to the importation of shipments into the United States.
- Product Description: An accurate description of the imported goods, including ingredients, contents, and their intended purpose.
- Manufacturer/Shipper Information: The importer must provide information about the manufacturer and shipper, along with their personal contact details.
- Country of Origin: The importer must provide information about the country where the product was produced or manufactured.
- Importer Information: According to the documentation, the importer must include their name, address, and contact information.
- Shipping Method
- Carrier Information: The importer must provide information about the carrier responsible for transporting the goods, such as the vessel name, flight number, or vehicle registration.
- Arrival Information: Expected date and time of arrival, port of entry, and shipping method.
- FDA Product Code: The importer must provide all required information as per FDA regulations.
3. When Do You Need a Prior Notice?

Businesses must submit Prior Notice before declaring to the FDA. In accordance with regulations, businesses are required to notify the FDA at least 15 days before the expected arrival of goods in the U.S. when submitting the notification via PNSI. If using ABI/ACS through U.S. Customs and Border Protection (CBP), the notification must be made at least 30 days in advance. Additionally, notifications must be completed within the following timeframes:
- No later than 2 hours before arrival for road transport;
- No later than 4 hours before arrival for rail transport;
- No later than 4 hours before arrival for air transport;
- No later than 8 hours before arrival for sea transport;
- Prior to dispatch for international mail;
- Follow the carrier’s schedule if a person is transporting or accompanying the goods.
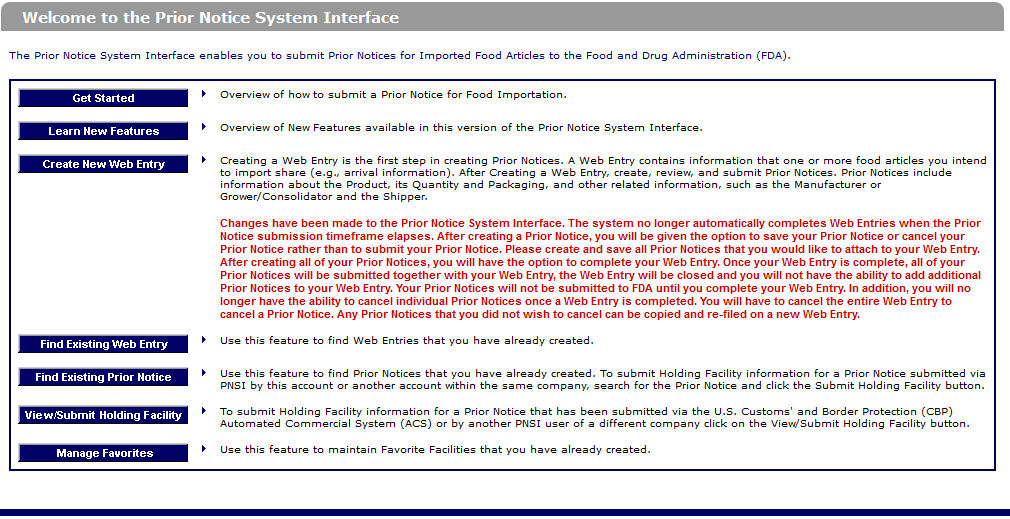
4. How to submit an FDA Prior Notice?
There are four steps to create a Prior Notice with the FDA as follows:
Step 1: Access PNSI
To begin, visit the official FDA Industry Systems Access website at www.access.fda.gov. On the homepage, locate the “Login” button in the upper right corner. Clicking this button will direct you to the login interface, where you can input your account information. This step is similar to using a key to unlock access to the FDA’s control system.
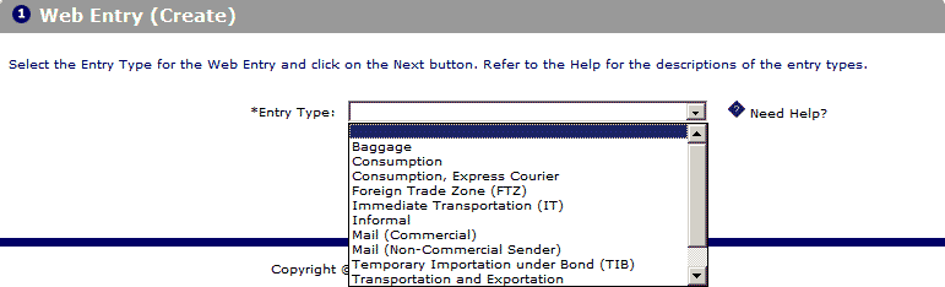
Step 2: Create a new Web entry
This is the first and most important step in the process of submitting a Prior Notice. On the main interface of the PNSI system, you will see the “Create Web Entry” button. Clicking this button will take you to the page where you can enter the basic information about the shipment.
On this page, you will find a dropdown menu to select the type of entry. There are two main options: “Mail (Commercial Purpose)” and “Mail (Non-commercial Sender).” You need to choose the type that matches your shipment and then click the “Next” button to continue. Selecting the correct type of entry will help the FDA easily classify and process the information.
Accordingly, you will sequentially fill in the relevant information, such as entry details, port of arrival, sender, importer, and more.
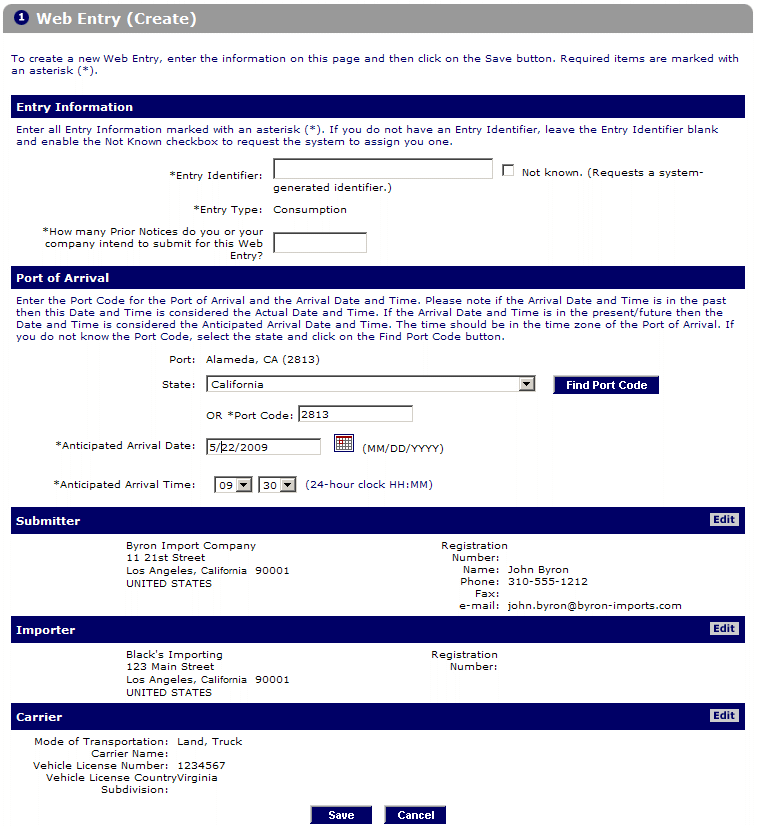
Step 3: Create and save the Prior Notice
Select the “Create Prior Notice” button at the bottom of the Web Entry page.
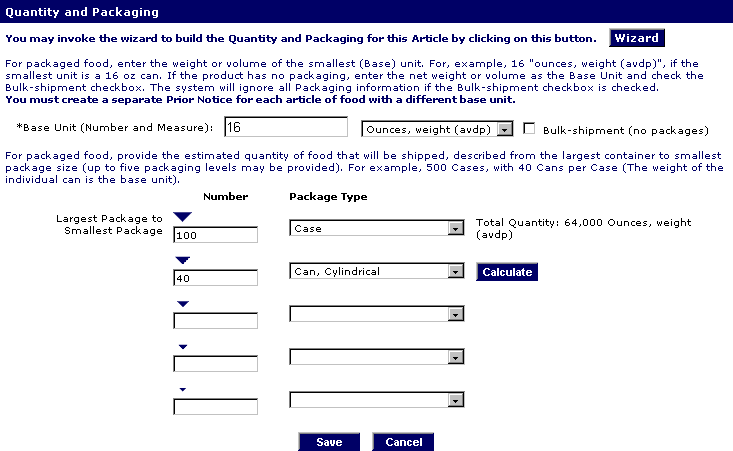
After that, enter the information to Create Prior Notice.
Step 4: Complete the Web entry
After completing all the steps, click to submit the Prior Notice to the FDA.
5. Risks of Not Submitting Adequate Prior Notice
If you do not submit the Prior Notice within the required timeframe, U.S. customs will not allow your goods to clear customs and will hold them at the port. This not only delays the agreed timeline with your partners, damaging your reputation, but also incurs storage costs, while the goods may be at risk of spoilage due to prolonged detention.
You must comply with the FDA’s Prior Notice regulations to ensure that your goods clear smoothly and quickly, avoiding unnecessary losses. If you import food without a complete Prior Notice, the port may deny entry to the shipment. According to FDA regulations, officials have the authority to impose bans, hold, deny, embargo, and prosecute depending on the severity of the failure to provide timely and accurate Prior Notice.
6. How UCC Assists with FDA Registration
UCC is a service provider specializing in consulting and filing declarations for businesses exporting food to the United States. Moreover, we are dedicated to helping you complete procedures quickly, accurately, and efficiently. Furthermore, we are always ready to support you with any related steps you may need. In addition, UCC also offers FDA compliance services for the cosmetics, food, medical device, and pharmaceutical industries. Contact UCC today for a detailed consultation. Our team is eager to discuss your needs and provide tailored guidance to support your business.
For more information: Food Label Consultants: Everything You Need to Know
Through this article, you have gained a clearer understanding of Prior Notice Meaning and the related procedures. UCC hope to have the opportunity to collaborate on upcoming projects!



