While many are familiar with FDA certification, some may not be aware that a vital component for obtaining FDA registration is the US agent. What is a FDA US agent, and what role do they play in this process? Join UCC as we delve into this topic in the following article.

1. Who Is The FDA US Agent?
According to FDA regulations, any foreign facility involved in manufacturing or handling imported devices must designate a US agent. The US agent can be an individual or an organization; however, they must reside in the United States or have a business location there.
Additionally, the US agent cannot use a P.O. Box as their address and cannot rely solely on answering services. Instead, they must have someone available to answer phone calls during normal business hours.

2. Why Do Businesses Need A US Agent?
As mentioned earlier, in the FDA registration process, businesses and manufacturing facilities are required to have a US agent to serve as a liaison between both parties. Therefore, it is essential for you to designate a US agent to assist with this process. Additionally, the information that needs to be provided to the FDA includes the name, address, phone number, fax number, and email. This information is submitted electronically through the FDA’s Unified Registration and Listing System.
Furthermore, once the US agent is designated, they will be required to complete an automated process and receive an email confirmation indicating their agreement to act as the US agent for the business. If the US agent declines the request or does not respond within 10 business days, the foreign facility will need to designate a different US agent to complete the legal requirements.
The responsibilities of an FDA US Agent include the following:
- Communication with FDA: Acting as a liaison, this agent conveys information and requests from the FDA to the foreign facility.
- Registration Support: They assist in completing the registration process and providing necessary information to the FDA.
- Ensuring Compliance: The agent monitors new regulations and informs the foreign facility to ensure compliance with FDA requirements.
- Receiving Notifications: They receive and forward notifications or documents from the FDA to the foreign facility.
- Assisting with Inspections: The agent supports the facility during FDA inspections by providing necessary information and documentation.
- Acting as a Representative: They ensure that the foreign facility has a US point of contact for any product-related issues.
The US agent will not have responsibilities related to reporting adverse events under the Medical Device Reporting regulations (21 CFR Part 803) or submitting Premarket Notification 510(k) (21 CFR Part 807).
For more information: Pre-Market Notification: A Guide to Obtaining FDA Approval
3. How To Connect With A US Agent To Complete FDA Certification?
When connecting with a US agent for FDA certification, businesses may face several challenges. These include finding a suitable representative and verifying their reliability. Assessing the agent’s experience and understanding of FDA regulations can also be difficult, especially with language barriers. Additionally, high service costs and ongoing changes in FDA regulations can create significant pressure for businesses.
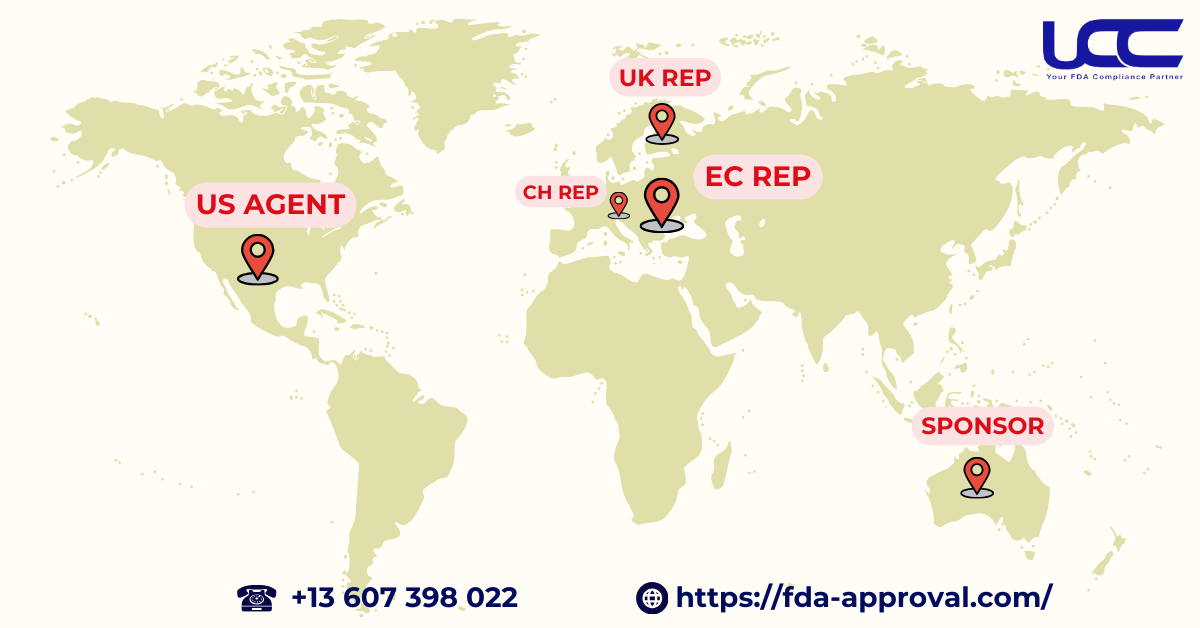
If you are seeking a professional and trustworthy provider of US agent services, UCC is the ideal choice. We offer representation not only in the United States but also in various regions, including Europe (ECREP), the United Kingdom (UK REP), Switzerland (CH REP), and Australia (Sponsor). Our expertise covers legal solutions in Food, Cosmetics, Pharmaceuticals, and Medical Devices. In addition, with a team of experienced professionals, UCC is dedicated to helping businesses navigate certification challenges and quickly access global markets.
4. Achievements Of UCC In The Role Of FDA US Agent
UCC has over 15 years of experience working with the US FDA, specializing in legal issues and communication with the agency. Moreover, our team of experts possesses extensive knowledge as well as rich experience. We are always ready to address and resolve complex legal requirements from the FDA. As a result, we promptly handle related issues such as warning notices, hold orders, and product returns at the port for our clients. To date, UCC has collaborated with numerous companies across various sectors. Notable partners include:
- VINACAFE
- Dae Myung Chemical Vietnam
- LA’P Pharmaceutical Co., Ltd.
- MEM-CO Medical Supplies and Equipment JSC
- And many other companies

If you are seeking a reliable partner to assist you with the certification process and manage legal issues related to the FDA US agent Services, including securing a US agent for FDA registration, contact UCC today. We are ready to support you on your journey to global success!



